Prof. Wendee Chiang
Tel : +886-4-23590121 ext 37311 ; Fax :+886-4-23599059. E-mail address: wdc@thu.edu.tw
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 3 ISSUE: 3
Page No: 305-317
Prof. Wendee Chiang
Tel : +886-4-23590121 ext 37311 ; Fax :+886-4-23599059. E-mail address: wdc@thu.edu.tw
Chingwen Liu1, Yicheng Wang2,3, Changchi Hsieh1, Wendee Chiang2*
1Department of Animal Science and Biotechnology, Tunghai University, 1727, Section 4, Taiwan Boulevard, Taichung 40704, Taiwan, R.O.C.
2Department of Food Science, Tunghai University, 1727, Section 4, Taiwan Boulevard, Taichung 40704, Taiwan, R.O.C.
3Best Center for Cellular Nutrition, Han-Sient Trading Co., Ltd, 13F., 665, Bannan Rd., Zhonghe Dist., New Taipei City 23557, Taiwan, R.O.C.
Sandra Aparecida Benite-Ribeiro(sandrabenite@ufg.br)
Ana Alzamendi(aalzamendi@imbice.gov.ar)
Elena Gangitano(Ele.gangitano@hotmail.it)
Nicola J A Scott(nicola.scott@otago.ac.nz)
Wen-Dee Chiang, Effects of guava leaf extract on glucose and lipid homeostasis in diet-induced insulin-resistant C57BL/6J mice(2018)SDRP Journal of Food Science & Technology 3(3)
In this study, the effects of aqueous guava leaf extract (GvEx) on insulin resistance were evaluated in diet induced insulin-resistant mice. Low (50 mg/kg), middle (150 mg/kg), and high (450 mg/kg) oral dose of GvEx were administrated to the high-fructose-high-fat fed insulin-resistant C57BL/6J mice. Our results showed that administration of high dose GvEx significantly enhanced the glucose tolerance, insulin sensitivity and increased the serum adiponectin content. Triglyceride and total cholesterol contents of blood and liver were all significantly decreased after treatment with GvEx. Further, western blot analysis revealed that high dose GvEx significantly enhanced the levels of peroxisome proliferator-activated receptor (PPAR)-γ and adiponectin in adipose tissue, as well as enhancing the phosphorylation of the AMP-activated protein kinase and PPARs in both liver and skeletal muscle tissues. In addition, the protein levels of phosphorylated protein kinase B and glucose transporter were also induced in liver and skeletal muscle tissues. In conclusion, GvEx can improve the disturbed glucose and lipid homeostasis in diet-induced insulin-resistant C57BL/6J mice.
Keywords: metabolic syndrome, diabetes mellitus, insulin resistance, guava leaf extract
Metabolic syndrome refers to the simultaneous presence of multiple metabolic abnormalities conditions, including abdominal obesity, impaired glucose tolerance, dyslipidemia, hypertension and other symptoms. These metabolic abnormalities are usually a sign of many chronic diseases, including type 2 diabetes, cardiovascular disease and hypertension. Metabolic syndrome of worldwide adult population is about 35%, which is 18.2% of Asian, 29.4% of Africa, 39.1% of Australia and 35% of American (Pudata and Konduru, 2011). According to the investigation of Taiwan Ministry of Health and Welfare showed that the prevalence of metabolic syndrome was 19.7% in adult population and increased with age. The results also noted that abdominal obesity will increase 50% chance of suffering from metabolic syndrome, while there are cases when coupled with abnormal blood pressure, the probability is as high as 75%. In addition, the subjects with metabolic syndrome will increase the risk of suffering from type 2 diabetes, hypertension, hyperlipidemia and cardiovascular disease for six times, four times, 3 times and 2 times, respectively (Ministry of Health and Welfare, Executive Yuan, Taiwan (R.O.C.), 2007).
Obesity is mainly because of excessive intake of energy resulting in excessive accumulation of body fat. As described by Bays et al., persons with diabetes, dyslipidemia and hypertension are respectively 84%, 79% and 82% have overweight or obese phenomenon, indicating the close correlation between obesity and metabolic syndrome (Bays et al., 2007). The formation mechanism of metabolic syndrome is not yet fully clarified, it is generally believed that may be associated with insulin resistance and abdominal obesity. The excessive free fatty acid inhibits insulin signaling pathways of muscle tissue, which resulting in the occurrence of insulin resistance and reducing glucose uptake in muscle cells (Eckel et al., 2005). In addition, excess body fat can promote the secretion of interleukin-6 and tumor necrosis factor-α, which will increase inflammatory response, inhibit insulin signal transduction and further leading to insulin resistance occurs (Dandona et al., 2004; Furukawa et al., 2004; Lazar, 2005; Kasuga, 2006; Shoelson et al., 2006). Based on the above, an excessive amount of body fat will lead to the occurrence of insulin resistance, indicating the importance of obesity amelioration for the prevention and/or improvement of metabolic syndrome.
Peroxisome proliferator-activated receptor γ (PPAR-γ), specifically and preferentially expressed in adipocytes, is mainly responsible for the regulation of insulin sensitivity, cell differentiation, inflammation and insulin sensitivity (Semple et al., 2006; Yang et al., 2008). PPAR-γ can be regulated by fatty acid, phytochemicals or drugs and affect the lipid and glucose metabolism related gene expression (Semple et al., 2006; Wang et al., 2014). Adiponectin is an adipose-specific plasma protein which are decreased in obese and type 2 diabetic subjects with insulin resistance. Thiazolidinediones (TZDs), PPAR-γ synthetic ligands, is a new class of antidiabetic drugs that improve insulin action. The administration of TZDs significantly increased both the plasma adiponectin concentrations and adiponectin mRNA expression of adipose tissues in insulin resistant rodents (Maeda et al., 2001). As described by Yoon et al., adiponectin increases fatty acid oxidation in skeletal muscle cells by sequential activation of AMP-activated protein kinase (AMPK), p38 Mitogen-activated protein kinase, and PPAR-α (Yoon et al., 2006).
Guava leaf has traditionally been used as a traditional folk herb for diabetes patients in Oriental countries. In recent years, the anti-hyperglycemic and anti-metabolic syndrome activities of guava leaves extract has been reported in animal models and human clinical studies (Yoshitomi et al., 2012a; Yoshitomi et al., 2012b; Guo et al., 2013; Khan et al., 2013; Oriaifo et al., 2014; Mathur et al., 2015). However, the mechanisms of guava leaf extracts on diabetes and/or metabolic syndrome improvement are only partially discussed in these researches. Our previous study has confirmed the effects of aqueous guava leaf extract (GvEx) on insulin resistance improvement via modulation of the insulin signaling pathway in high glucose-induced insulin-resistant mouse FL83B cells (Liu et al., 2015a). In the present study, the mechanisms of GvEx on metabolic syndrome improvement was comprehensively evaluated in hepatic, white adipose, and skeletal muscle tissues of high-fructose-high-fat fed insulin-resistant C57/BL6J mice.
2.1. Plant material and GvEx preparation.
Plant material and GvEx preparation were described in our previous study (Liu et al., 2014). Briefly, Jen Ju Pa leaves were collected at period between the initial appearance and the visible opening of flower buds. The plant materials were taxonomically identified and the data have been deposited at the Fengshan Tropical Horticultural Experiment Branch, Taiwan Agricultural Research Institute Council of Agriculture, Executive Yuan (FTHA000282). Guava leaf extraction were performed with the optimal extraction conditions which derived from a response surface methodology. The freeze-dried GvEx was stored at -80°C and the quality of GvEx was evaluated by total phenolic content (26.12%) and chromatographic profile of the phenolic components including catechin, epicatechin, gallic acid, quercetin, chlorogenic acid, epigallocatechin gallate and caffeic acid (Liu et al., 2014).
2.2. Animals.
Ten-week-old male C57BL/6J mice were obtained from Taiwan NLAC (Taipei, Taiwan). All mice were housed in a climate-controlled room with 20-24°C temperature, 40-60% humidity, 12-hour light and dark cycle, and free access to normal chow and water at the agricultural college of Tunghai university. After one week, mice were randomly allotted to 6 groups including a normal control group (normal chow, not treated; N), an experimental control group (high-fructose-high-fat fed, not treated; C), a positive control group (high-fructose-high-fat fed, acarbose gavage administered as 61 mg/kg/day; P), a low dose group (high-fructose-high-fat fed, GvEx gavage administered as 50 mg/kg/day; TL), a middle dose group (high-fructose-high-fat fed, GvEx gavage administered as 150 mg/kg/day; TM) and a high dose group (high-fructose-high-fat fed, GvEx gavage administered as 450 mg/kg/day; TH). According to the result of our previous in vitro study, 200 and 400μg/ml GvEx (ED50 in vitro), respectively, showed significantly increased the rate of glucose uptake in both normal and insulin-resistant cells (Liu et al., 2015). Therefore, the effective doses (ED50 in vivo) for animal model were 43.58 and 61.88 mg/kg, respectively, after calculation with the formula log (ED50 in vivo) = 0.506 x log (ED50 in vitro) + 0.475 (Popiolkiewicz et al., 2005; Kitagaki et al., 2006). The low dose (50mg/kg) was the average of effective dosages. The middle dose (150mg/kg) was three times of low dose and high dose (450mg/kg) was nine times. The dose of acarbose was in accordance with the maximum effective dose of 61.88 mg/kg GvEx for animal model.
2.3. Induction of insulin resistance and GvEx treatment.
To induce insulin resistance, the C, P, TL, TM, TH groups were fed with high fat diet (fat content, 60 Kcal%, research diet) and 30% (w/v) fructose was added to the drinking water. These mice were gavage administrated with their relatively treatment at the beginning of high-fructose-high-fat feeding. The extract was dissolved in drinking water for the oral gavage. The N group was maintained on regular chow diet and plain water (untreated). At the end of the 10-week period, all animals were placed on a 14 h fast, sacrificed by cervical dislocation and exsanguination prior to sample collection. The tissues and serum were flash-frozen in liquid nitrogen and stored at -80 °C until further analyses were carried out.
2.4. Oral glucose tolerance test (OGTT).
After 14 h fasting, animals were subjected to OGTT on week 8. Briefly, individual mice were administered orally with 1 g/kg BW of glucose and their blood glucose levels were measured at 0, 30 60, 90, and 120 min post glucose challenge. The area under curve (AUC) over the blood glucose levels were calculated by the trapezoidal rule: [(gluc0min+ gluc30min) ×30/2] + [(gluc30min+gluc60min) ×30/2] + [(gluc60min+gluc90min) ×30/2] + [(gluc90min+gluc120min) ×30/2] (van Hoek et al., 2009).
2.5. Biochemical measurement.
The concentrations of serum triglycerides (TG), cholesterol (CHOL), glutamate oxaloacetate transaminase (GOT), and glutamate pyruvate transaminase (GPT) levels were determined by regular biochemistry assays using the corresponding commercial enzyme kit on a Biochemical Analyzer (F. Hoffmann-La Roche LTD., Basel, Switzerland). Serum insulin and adiponectin concentrations were measured using mouse insulin (Mercodia Inc., Uppsala, Sweden) and adiponectin (Abcam, Cambridge, United Kingdom) ELISA kits, respectively.
2.6. Homeostatic model assessment-insulin resistance (HOMA-IR).
The HOMA model is a method used to quantify insulin resistance and β-cell function from fasting serum glucose and insulin concentrations. The model has been widely used since it was first published (Wallace et al., 2004). After 14 h fasting, the blood glucose and insulin concentrations of mice were obtained and used to HOMA-IR calculation. The formula was as follows: HOMA-IR = [fasting insulin (μU/mL) x fasting glucose (mmol/L)] / 22.5.
2.7. Hematoxylin-eosin (HE) stain
HE stain is the most widely used method in histology and histopathology analysis. It is a relatively simple method to demonstrate a wide range of cytoplasmic, nuclear, and extracellular matrix features on paraffin or frozen sections. Liver tissues were harvested and fixed in 10% neutral formalin, embedded in paraffin, cut into 6-μm-thick sections on slides, and stained with hematoxylin and eosin. Before immunostaining, deparaffinization was performed in xylene and graded ethanol to distilled water. After deparaffinization, slides were stained in hematoxylin for 1~3 min followed by a running water wash. After 15 mins wash, the slides were stained in eosin for 5 secs. Slides were dehydrated, cleared and mounted for examination by light microscopy.
2.8. Hepatic triglyceride (TG) and cholesterol (CHOL) analysis.
Liver tissues were homogenized in an ice-cold phosphate-buffered saline (PBS) solution. Hepatic lipids were extracted following an adaptation of published methods (Folch et al., 1957). Briefly, homogenate was extracted with a 1:2 (v:v) mixture of methanol/chlororform, with vortexing to mix thoroughly. The samples were then spun at 4,200 g at 4°C for 10 min. Organic (lower) phase was dried under vacuum condition and the TG and CHOL levels were measured by regular biochemistry assays using the corresponding commercial enzyme kit on a Biochemical Analyzer (F. Hoffmann-La Roche LTD., Basel, Switzerland).
2.9. Western blot analysis.
The liver, white adipocytes and skeletal muscle tissues were homogenized with ice cold lysis buffer (0.05 M Tris-HCl, pH 7.4, 0.15 M·NaCl, 0.25% deoxycholic acid, 1% NP-40, 1 mM EDTA) at a ratio of 100 mg tissue/1 mL buffer, respectively. Homogenates were then centrifuged at 27,000 x g for 10 min at 4°C. The supernatant was isolated and immunoblotting and was performed as described previously (Liu et al., 2015a). The supernatant was used for the determination of protein abundance, including phospho-tyrosine of AMPK (p-AMPK (Thr)), phosphor-serine of protein kinase B (p-Akt (Ser)), PPAR-α, PPAR-γ, PPAR-δ, adiponectin, glucose transporter (Glut) 2, and Glut 4. β-Actin was used as an internal control to ensure adequate sample loading for all lands. The bound antibodies were detected with horseradish peroxidase-conjugated secondary antibodies, followed by enhanced chemiluminescence substrates (Millipore, Billerica, MA).
2.10. Statistical assay.
All experiments were performed at least in triplicate, and the results are expressed as the mean + SD. Statistical analysis was done by one-way ANOVA with Tukey’s post hoc test. A p value less than 0.05 was considered statistically significant.
3.1. Effects of GvEx on body and organ weights of experimental animals.
Supplementation of GvEx significantly decreased the body weights of high-fructose-high-fat fed mice. As shown in Figure 1, mean body weights were no difference between all groups at the beginning of the study. After ten weeks of treatment, mean body weights were significantly lowest in TH (30.8 ± 2.2 g), followed by TM (31.8 ± 2.7 g) and P (32.3 ± 1.9 g) as compared to C (34.4 ± 2.3 g), suggesting the beneficial effect of GvEx on lowering body weight (Figure 1). In addition, treated with high dose GvEx significantly decreased the weights of epididymal fat and white adipose tissue (sum of perirenal fat, abdominal fat, and epididymal fat) in high-fructose-high-fat fed mice (Table 1). A previous report indicated that in high-fat diet induced obese mice, treated with different dose of Psidium guajava leaves extract (LD: 50 mg/kg; MD: 100 mg/kg; HD: 200 mg/kg) decreased the body weight and visceral fat accumulation, and the lowering activity was dose-depended (Li et al., 2012). SHRSP.Z-Leprfa/IzmDmcr (SHRSP/ZF) rats, a metabolic syndrome animal model, which were administrated with 2 g/kg/day guava leaf extract for 6 weeks. The results indicated that administration of guava leaf extract significantly reduced body weight and adipose weight as compared to control group (Yoshitomi et al., 2012a). Based on these results, we propose that GvEx may be a purely nature component with anti-obesity and lipid lowering effects. Thus, extracts from guava leaves may be a purely nature component with anti-obesity and lipid lowering effects.
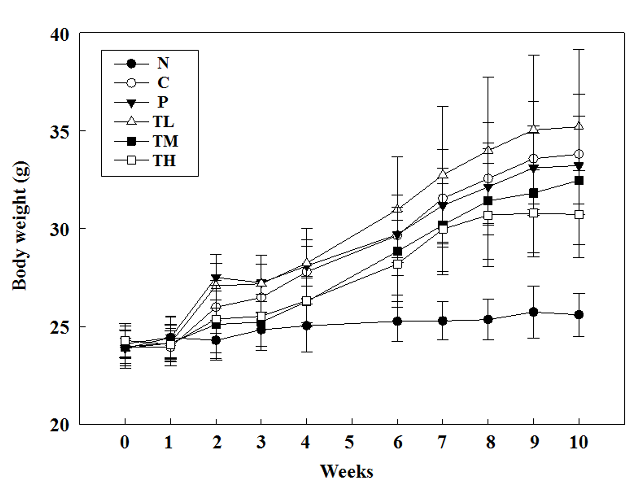
Figure 1: The effect of guava leaf extract on body weight in experimental animals. Mean body weights were significantly lowest in TH (30.8 ± 2.2 g), followed by TM (31.8 ± 3.1 g) and P (32.83 ± 2.0 g) as compared to C (34.2 ± 2.4 g) at week 10.
Table 1. The effect of guava leaf extract on body weight, liver weight, kidney weight, spleen weight, perirenal fat weight, abdominal fat weight, epididymal fat weight and total white adipose weight in animals of each group.
|
|
N |
C |
P |
TL |
TM |
TH |
|
Body weight (g) |
25.7±1.32a |
34.4±2.26d |
32.3±1.88c |
36.3±2.08e |
31.8±2.65c |
30.8±2.22b |
|
Liver (g) |
0.94±0.07a |
1.06±0.11bc |
1.06±0.06bc |
1.13±0.13c |
1.09±0.11bc |
1.01±0.09ab |
|
Kidney (g) |
0.30±0.06 |
0.31±0.15 |
0.30±0.03 |
0.27±0.07 |
0.31±0.05 |
0.28±0.05 |
|
Spleen (g) |
0.05±0.01 |
0.06±0.01 |
0.06±0.01 |
0.06±0.01 |
0.07±0.02 |
0.06±0.02 |
|
Perirenal fat (g) |
0.15±0.19a |
0.69±0.22b |
0.62±0.29b |
0.80±0.25b |
0.57±0.29b |
0.53±0.23b |
|
Abdominal fat (g) |
0.31±0.11a |
1.37±0.45b |
1.33±0.57b |
1.35±0.64b |
1.25±0.54b |
1.17±0.39b |
|
Epididymal fat (g) |
0.45±0.37a |
1.70±0.43c |
1.32±0.49bc |
2.06±0.44d |
1.58±0.37bc |
1.27±0.36b |
|
White adipose tissue (g) |
0.94±0.70a |
3.75±1.02cd |
2.93±1.22bc |
4.45±1.02d |
3.42±0.97bc |
2.77±0.69b |
N: normal control, C: experimental control, P: positive control, TL: low dose, TM: middle dose, TH: high dose.
Values with different letters on each column are significantly different (p < 0.05;n > 8).
3.2. Effects of GvEx on the improvement of glucose tolerance and insulin resistance in experimental animals.
While there was no significant difference in the dynamics of blood glucose levels following oral glucose loading on day 28 post treatment within high-fat-high-fructose fed groups, the levels of blood glucose in the TH group were significantly lower than that in the C group at 60 (219.1 ± 66.0 vs. 316.0 ± 85.5 mg/dL, p = 0.006), 90 (199.0 ± 57.5 vs. 247.7 ± 47.9 mg/dL, p = 0.033), and 120 minutes (191.0 ± 44.9 vs. 231.5 ± 45.9 mg/dL, p = 0.039) post glucose loading on day 56. In addition, the levels of blood glucose in the P group were significantly less than that of the C group at 60 minutes (316.0 ± 85.5 vs. 235.3 ± 34.3 mg/dL, p = 0.004) after oral glucose loading (Figure 2A). The area under the AUC is dependent on the rate of elimination of the administered glucose from the body. The AUC is inversely proportional to the clearance of the glucose. It can be used to estimate a change in the clearance of blood sugar in specific clinical conditions, such as metabolic syndrome and/or diabetes. As a result, the AUC values over the 120 minutes of glucose challenge in the TH group were significantly lower than that of the C group (27818 ± 7046 vs. 34075 ± 5190, p = 0.014), suggesting the effects of GvEx on improvement of glucose tolerance (Figure 2B). HOMA is a method for evaluating IR and beta-cell function from blood glucose and insulin concentrations. The HOMA-IR is a widely used clinical and epidemiological tool for the assessment of insulin resistance; higher values are associated with higher incidence of metabolic syndrome, and lower insulin sensitivity (Qu et al., 2011). High-fructose-high-fat feeding caused a significant elevation (p < 0.05) in fasting blood sugar levels (Figure 3A) and HOMA-IR (Figure 3C) and didn’t affect serum insulin level (Figure 3B) as compared to N group. Oral administration of high dose GvEx in the last 10 weeks reduced serum insulin levels (Figure 3B) and HOMA-IR (Figure 3C), suggesting the effectiveness of GvEx that improve insulin sensitivity.
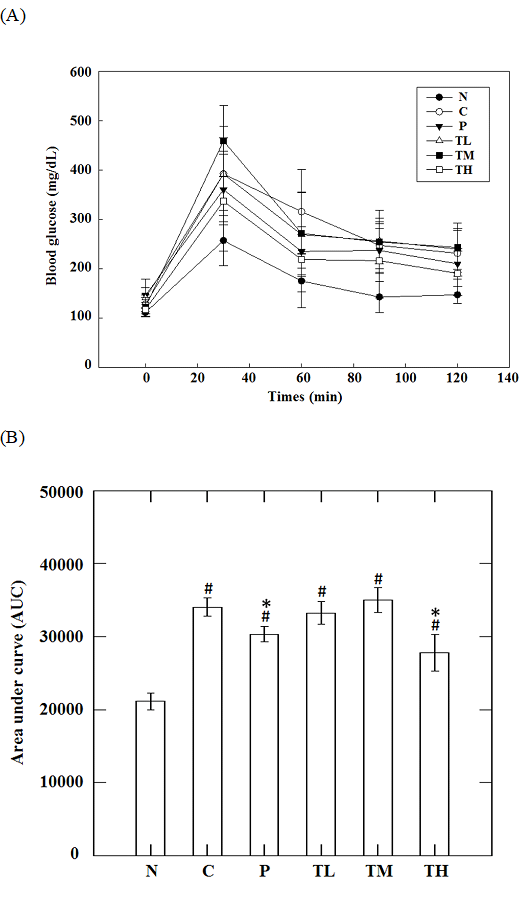
Figure 2 : (A) Oral glucose tolerance test curve and (B) averaged area under curve on serum glucose level in animals. #, significantly different (p < 0.05) from N group; *, significantly different (p < 0.05) from C group.
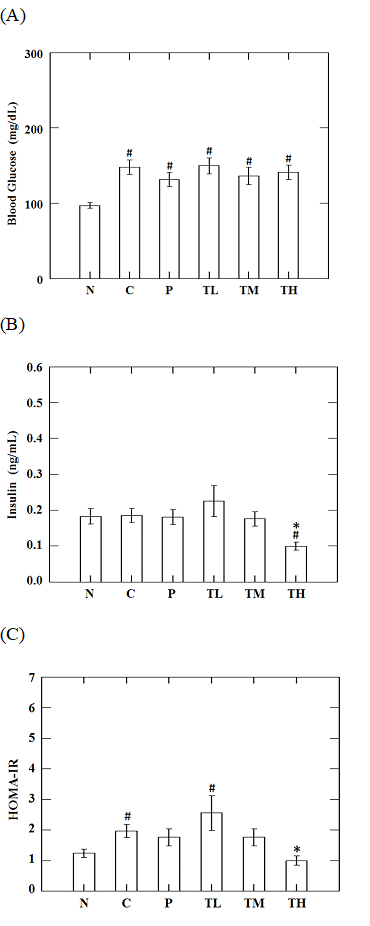
Figure 3: Effects of guava leaf extract on fasting serum (A) glucose, (B) insulin, and (C) insulin sensitivity of animals in each group. #, significantly different (p < 0.05) from N group; *, significantly different (p < 0.05) from C group.
Our previous studies indicate that polyphenols of GvEx may effectively inhibit the activities of α–amylase and α–glucosidase (Liu et al., 2014; Liu et al., 2015b), so that the amount of glucose can be released slowly to avoid postprandial blood sugar rise sharply, thus preventing the excessive secretion of insulin. Furthermore, GvEx can also increase the sensitivity of insulin receptor, including phosphorylated insulin receptor substrate 1, phosphoinositide 3-kinase, phosphorylated protein kinase B, etc., that will activate the insulin signaling pathways and promote glucose metabolism (Guo et al., 2013; Liu et al., 2015a). As described by Mathur et al., to induce insulin resistance, the 4 weeks old male rats were provided with 15% (w/v) fructose as drinking water, while parallel treatment groups were administered with guava leaf extract for 8 weeks. After treatment period, the levels of blood glucose were significantly lower in the dose of 250 (PG1) and 500 (PG2) mg/kg/day guava leaf extract treated group than that in the control group at 120 minutes (112.6 ± 28.6 and 93.4 ± 18.2 vs. 152.2 ±16.2 mg/dL) after oral glucose loading. The AUC of OGTT test in control, PG1 and PG2 groups was 36444 ± 1110, 20208 ± 1023 and 18486 ± 1005, respectively. The AUC was significantly less (p < 0.001) in PG1 and PG2 groups as compared to control group. In addition, fructose intake clearly led to over 3-fold increase in HOMA-IR value, and treatment with guava leaf extract showed a significant decrease in plasma insulin concentration, HOMA-IR values, and fasting blood sugar control group (Mathur et al., 2015). In the Deguchi et al. (2000) study, 16 of subjects with pre-diabetes and mild diabetes were administrated with 190 ml of Guava Leaf Tea at every meal for 12 weeks. Results showed that the fasting blood sugar level were more pronounced decrease in the pre-diabetic subjects (p = 0.06, n=7). Moreover, the levels of blood insulin and HOMA-IR values were significantly decreased in all subjects (Deguchi et al., 2000).
These results revealed that guava leaf extract can be used to alleviate glycemic response, including decreased blood sugar concentration and/or AUC. In addition, guava leaf extract that are effective in improving insulin resistance status of diabetic animals.
3.3. Effects of GvEx on lipid metabolism in experimental animals.
High fructose and/or high fat intake disturbs glucose metabolism and leads to a significantly increased rate of lipogenesis and TG synthesis in liver, and TG levels in circulation. These elevated TG levels can induce fatty liver, adipose accumulation, and decrease the response to insulin thereby causing IR. Fructose and/or fat-induced insulin resistant statuses are commonly characterized by the metabolic dyslipidemia, including elevated levels of circulating free fatty acid, TG, or CHOL levels (Basciano et al., 2005; Ginsberg et al., 2005; Liu et al., 2015c). In this study, high-fructose-high-fat fed mice showed insulin resistance and impaired glucose tolerance accompanied by increased TG and CHOL levels in circulation and liver, and accumulation of adipose tissue. High dose GvEx treatment significantly decreased the body weights (Figure 1) and adipose weights (Table 1) as compared to C group. In addition, administration of high dose GvEx indicated a significant decrease in the accumulation of TG and CHOL in the liver (Figure 4C, D) as well as in the circulation (Figure 4A, B).
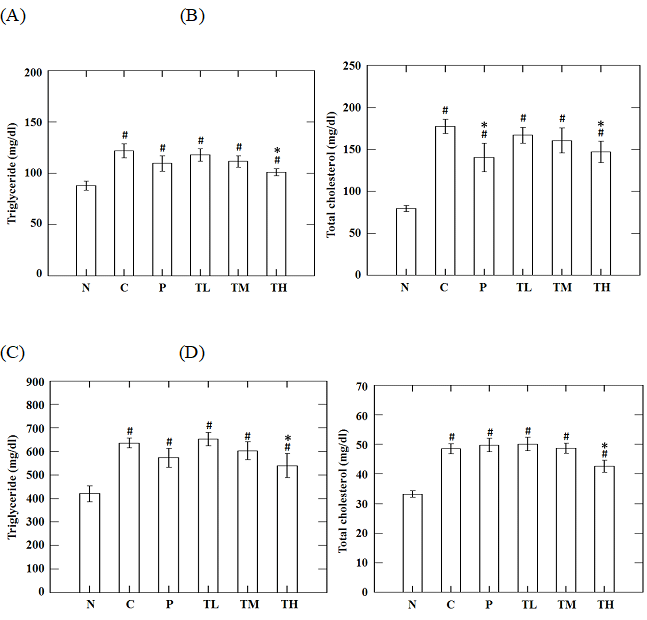
Figure 4: Effects of guava leaf extract on serum (A) triglyceride, (B) total cholesterol and liver (C) triglyceride, (D) total cholesterol in animals of each group. #, significantly different (p < 0.05) from N group; *, significantly different (p < 0.05) from C group.
PPAR-γ and adiponection are the factors that regulate pathways of lipid and carbohydrate metabolism (Yamauchi et al., 2001; Kota et al., 2005). In our study, the protein levels of PPAR-γ and adiponection were analyzed in epididymal fat tissue. The results showed a significant reduction in PPAR-γ and adiponection levels of high-fructose-high-fat fed mice as compared to the levels in N group. Administration of high dose GvEx significantly elevated the PPAR-γ and downstream adiponection levels (Figure 7A). Adiponectin, an insulin sensitizer that secreted by white adipose tissue, has been shown to activate fatty acid oxidation and enhance insulin sensitivity through the stimulation of AMPK in the peripheral tissues (Kadowaki and Yamauchi, 2005). Biochemical analysis of blood serum from the experimental animals showed that the concentrations of adiponectin, which was low under insulin-resistant state, were enhanced by high dose treatment with GvEx (Figure 6). Adiponectin downstream effectors, including AMPK and PPARs, were assayed in liver and skeletal muscle tissues. As shown in Figure 7B, high-fructose-high-fat diet reduced the pAMPK and PPAR-α levels in liver, and supplemented with middle and high dose of GvEx will ameliorate it. Similar results were also observed in skeletal muscle tissue that protein levels of pAMPK and PPAR-δ were restored by GvEx (Figure 7C). According to the assays on adiponectin downstream effectors, data suggest that the adiponectin signaling is intact up to pAMPK and PPARs, thereby may stimulate the activation of fatty acid oxidation and decrease tissue TG content. Moreover, histological analysis of liver sections from experimental animals also showed that the accumulation of lipid droplets was drastically decreased in TH group as compared to C group (Figure 5).
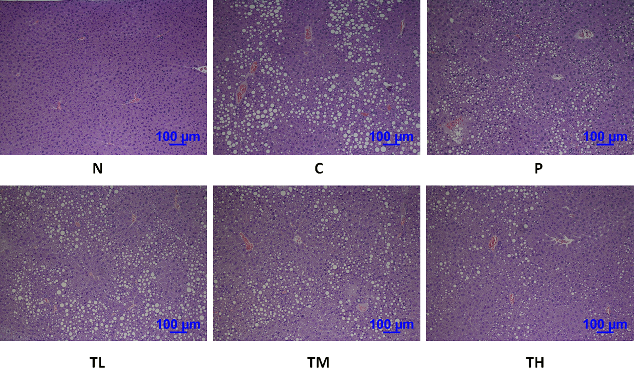
Figure 5: Hematoxylin-eosin staining of liver sections from mice with or without GvEx treatment. The accumulation of lipid droplets was drastically decreased in TH group as compared to C group.
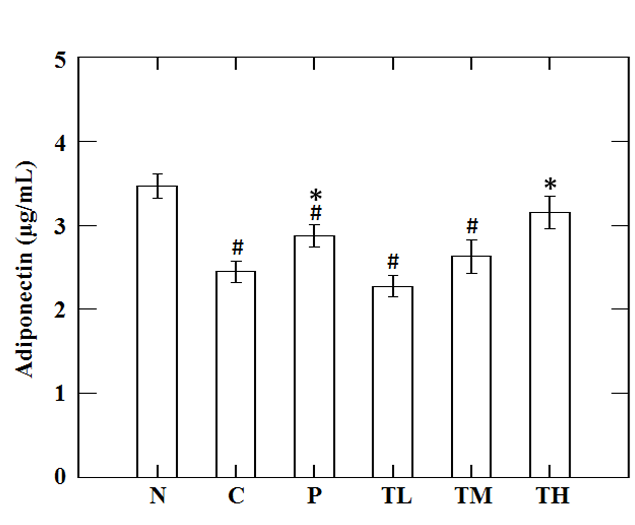
Figure 6: Effect of guava leaf extract on serum adiponectin in animals of each group. #, significantly different (p < 0.05) from N group; *, significantly different (p < 0.05) from C group.
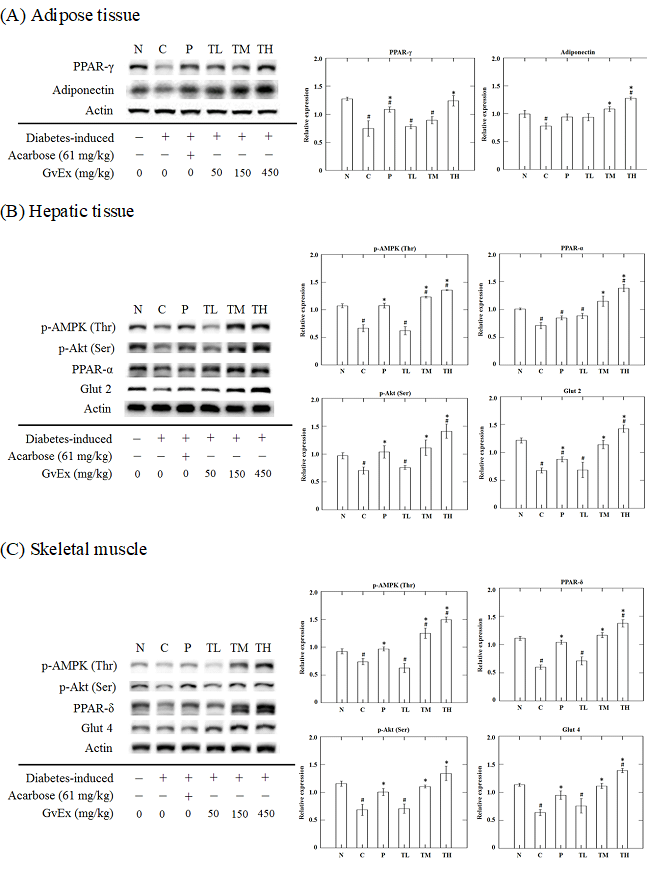
Figure 7: Effects of guava leaf extract on glucose and fat metabolism related signal transduction mechanism in (A) adipose tissue, (B) hepatic tissue, and (C) skeletal muscle of diet induced insulin resistant mice. #, significantly different (p < 0.05) from N group; *, significantly different (p < 0.05) from C group.
3.4. Effects of GvEx on the improvement of metabolic syndrome.
Several studies have indicated that high fructose and/or high fat diet can induce insulin resistance, hyperinsulinemia, hyperglycemia, and even the diabetes. The main reasons which cause the occurrence of these symptoms include: (1) Diet-induced increase in blood triglyceride levels would decrease the number of activated insulin receptors, thereby reducing insulin sensitivity. (2) Chronic inflammation and high oxidative stress status caused by high fructose and/or high fat diet would reduce the levels of phosphorylated insulin receptor, and decrease the amount of adiponectin expression in adipose tissue, thus lowering insulin sensitivity and disturbing homeostasis of glucose and lipid metabolism (Qin et al., 2003; Wellen and Hotamisligil, 2005; Ye et al., 2007). Furthermore, visceral fat tissue is known to actively release substantial amounts of free fatty acid than the subcutaneous fat tissue, that can lead to insulin resistance and metabolic abnormalities, and thus resulting in high blood sugar levels and other risk factors for metabolic syndrome (Schetman et al., 1989; Mann et al., 1997; Mori et al.,1997). Therefore, to reduce the concentration of triglycerides in the blood, increasing the degree of phosphorylated insulin receptor, activating insulin signal transduction, enhancing adiponectin abundance in adipose tissue, and reducing the amount of visceral fat would help prevent or ameliorate the metabolic syndrome status.
In our study, high-fructose-high-fat fed animals showed insulin resistance and impaired glucose tolerance accompanied by dyslipidemia. In addition, the adiponectin concentrations in circulation and the abundances of glucose and lipid metabolism-related signals in adipose, liver and skeletal muscle tissues were all drastically decreased. After 10- week treatment, the metabolic syndrome status of high-fructose-high-fat fed mice were ameliorated by high dose GvEx. Our previous study has demonstrated that GvEx could improve high-glucose induced insulin resistant conditions in mouse FL83B cells via enhancing the levels of signals involved in insulin transduction, including phospho-tyrosine of insulin receptor, phospho-tyrosine of insulin receptor substrate, p85 regulatory subunit of phospho-inositide 3 kinase, p-Akt (Ser), Glut 2, and glycogen synthase (Liu et al., 2015a). These results were also confirmed in the present study by a diet-induced insulin resistant animal models. Data from experimental animals showed that the protein levels of p-Akt (Ser) and Glut were all significantly induced in both liver and skeletal muscle tissues as compared to C group (Figure 7B, C). According to the above results, we suggested that GvEx could improve the high-fructose-high-fat diet induced metabolic syndrome status via activating lipid and glucose metabolism-related signaling in adipose, liver and skeletal muscle tissues (Figure 8).
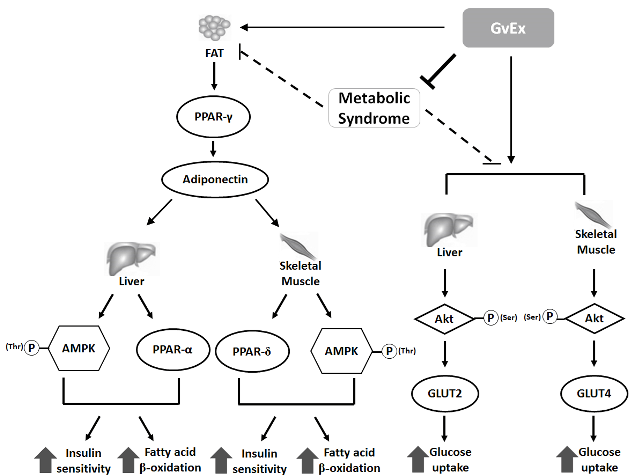
Figure 8: Schematic model of GvEx on modulating glucose and lipid homeostasis in diet-induced insulin-resistant C57BL/6J mice.
In conclusion, we evaluated the effects of GvEx on improvement of metabolic syndrome status in high-fructose-high-fat diet-induced insulin-resistant C57BL/6J mice. Our results showed that administration of high dose GvEx had preventive effects against the accumulation of lipid and ameliorated insulin resistance. The mechanism for this action was by the enhancing of glucose and lipid metabolism-related signals in adipose, liver and skeletal muscle tissues.
The authors are grateful for the financial support for this research by the Tunghai University of Taiwan R.O.C., under the project of “Global Research and Education on Environment and Society (GREEnS)” and Grand No. GREEnS 4-3.
Basciano, H., L. Federico, and K. Adeli. 2005. Fructose, insulin resistance, and metabolic dyslipidemia. Nutr. Metab. 2: 5. PMid:15723702
View Article PubMed/NCBIBays, H. E., R. H. Chapman, and S. Grandy. 2007. The relationship of body mass index to diabetes mellitus, hypertension and dyslipidaemia: comparison of data from two national surveys. Int. J. Clin. Pract. 61: 737-747. PMid:17493087
View Article PubMed/NCBIDandona, P., A. Aljada, and A. Bandyopadhyay. 2004. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 25: 4-7. PMid:14698276
View Article PubMed/NCBIDeguchi, Y., K. Osada, O. Chonan, K. Kobayahsi, A. Oohashi, T. Kitukawa, M. Watanuki, M. Ooni, K. Nakajima, and Y. Hata. 2000. Effectiveness of consecutive ingestion and excess intake of guava leaves tea in human volunteers. J. Jap. Counc. Adv. Food. Ingredients. Res. 1: 19-28.
Eckel, R. H., S. M. Grundy, and P. Z. Zimmet. 2005. The metabolic syndrome. Lancet 365: 1415-1428. 66378-7
View ArticleFolch, J., M. Lees, and G. H. S. Stanley. 1957. A simple method for the isolation and purification of total lipids from animal tissue. J. Biol. Chem. 226: 497. PMid:13428781
PubMed/NCBIFurukawa, S., T. Fujita, and M. Shimabukuro. 2004. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest. 114: 1752-1761. PMid:15599400
View Article PubMed/NCBIGinsberg, H. N., Y. L. Zhang, and A. Hernandez-Ono. 2005. Regulation of plasma triglycerides in insulin resistance and diabetes. Arch. Med. Res. 36: 232-240. PMid:15925013
View Article PubMed/NCBIGuo, X., H. Yoshitomi, M. Gao, L. Qin, Y. Duan, W. Sun, T. Xu, P. Xie, J. Zhou, L. Huang, and T. Liu. 2013. Guava leaf extracts promote glucose metabolism in SHRSP.Z- Leprfa/Izm rats by improving insulin resistance in skeletal muscle. BMC Complement Altern. Med. 1: 13-52.
View ArticleKasuga, M. 2006. Insulin resistance and pancreatic {beta} cell failure. J. Clin. Invest. 116: 1756-1760. PMid:16823472
View Article PubMed/NCBIKadowaki, T., and T. Yamauchi. 2005. Adiponectin and adiponectin receptors. Endocr. Rev. 26: 439-451. PMid:15897298
View Article PubMed/NCBIKhan, H. B., R. Shanmugavalli, D. Rajendran, M. R. Bai, and S. Sorimuthu. 2013. Protective effect of Psidium guajava leaf extract on altered carbohydrate metabolism in streptozotocin- induced diabetic rats. J. Diet Suppl. 10: 335-344. PMid:24237189
View Article PubMed/NCBIKota, B. P., T. H. Huang, and B. D. Roufogalis. 2005. An overview of biological mechanisms of PPARs. Pharmacol. Res. 51: 85-94. PMid:15629253
View Article PubMed/NCBILazar, M. A. 2005. How obesity causes diabetes: Not a tall tale. Science 307: 373-375. PMid:15662001
View Article PubMed/NCBILi, X., L. Yang, J. F. Wang, X. F. Li, and G. P. Cai. 2012. Effects of extracts from Psidium guajava leaves on anti-obesity and lipid lowering activity in high-fat diet induced obese mice. Progress in Modern Biomedicine 31: 6001-6005.
Liu, C. W., Y. C. Wang, H. C. Lu, and W. D. Chiang. 2014. Optimization of ultrasound-assisted extraction conditions for total phenols with anti-hyperglycemic activity from Psidium guajava leaves. Process Biochem. 49: 1601-1605.
View ArticleLiu, C. W., Y. C. Wang, C. C. Hsieh, H. C. Lu, and W. D. Chiang. 2015a. Guava (Psidium guajava Linn.) leaf extract promotes glucose uptakeand glycogen accumulation by modulating the insulin signaling pathway in high-glucose-induced insulin-resistant mouse FL83B cells. Process Biochem. 50: 1128-1135.
View ArticleLiu, C. W., Y. C. Wang, C. Y. Huang, H. C. Lu, and W. D. Chiang. 2015b. Optimization extraction conditions with ultrasound for anti-hyperglycemic activities from Psidium guajava leaf. Food Sci. Technol. Res. 21: 615-621.
View ArticleLiu, Z., I. Y. Patil, T. Jiang, H. Sancheti, J. P. Walsh, B. L. Stiles, F. Yin, and E. Cadenas. 2015c. High-fat diet induces hepatic insulin resistance and impairment of synaptic plasticity. PLoS One 10: e0128274. PMid:26023930
View Article PubMed/NCBIMaeda, N., M. Takahashi, T. Funahashi, S. Kihara, H. Nishizawa, K. Kishida, H. Nagaretani, M. Matsuda, R. Komuro, N. Ouchi, H. Kuriyama, K. Hotta, T. Nakamura, I. Shimomura, and Y. Matsuzawa. 2001. PPARgamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes 50:2094-2099. PMid:11522676
View Article PubMed/NCBIMann, N., A. Sinclair, M. Pille, L. Johnson, G. Warrick, E. Reder, and R. Lorenz. 1997. The effect of short-term diets rich in fish, red meat, or white meat on thromboxane and prostacyclin synthesis in humans. Lipids 32: 635-644. PMid:9208393
View Article PubMed/NCBIMathur, R., S. Dutta, T. Velpandian, and S. R. Mathur. 2015. Psidium guajava Linn. leaf extract affects hepatic glucose transporter-2 to attenuate early onset of insulin resistance consequent to high fructose intake: An experimental study. Pharmacognosy Res. 7: 166-175. PMid:25829790
View Article PubMed/NCBIMinistry of Health and Welfare, Executive Yuan, Taiwan (R.O.C). 2007. The metabolic syndrome criteria. TopicArticle.aspx?No=200712250123&parentid=200712250023.
View ArticleMori, T. A., L. J. Beilin, V. Burke, J. Morris, and J. Ritchie. 1997. Interactions Between Dietary Fat, Fish, and Fish Oils and Their Effects on Platelet Function in Men at Risk of Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 17: 279-286. PMid:9081682
View Article PubMed/NCBIOriaifo, S. E., E. K. Omogbai, and N. I. Oriaifo. 2014. Chronic administration of psidium guajava tea lowers free fatty acid levels in the metabolic syndrome. Int. Res. J. Pharm. Pharmacol. 4: 1-5.
Pudata, V., and J. Konduru. 2011. Metabolic syndrome in Endocrine System. J. Diabetes Metab. 2: 163-168.
View ArticleQin, B., M. Nagasaki, M. Ren, G. Bajotto, Y. Oshida, and Y. Sato. 2003. Cinnamon extract (traditional herb) potentiates in vivo insulin-regulated glucose utilization via enhancing insulin signaling in rats. Diabetes Res. Clin. Pract. 62: 139-148. 00173-6
View ArticleQu, H. Q., Q. Li, A. R. Rentfro, S. P. Fisher-Hoch, and J. B. McCormick. 2011. The Definition of Insulin Resistance Using HOMA-IR for Americans of Mexican Descent Using Machine Learning. PLoS ONE 6: e21041. PMid:21695082
View Article PubMed/NCBISemple, R. K., V. K. Chatterjee, and S. O'Rahilly. 2006. PPARγ and human metabolic disease. J. Clin. Invest. 116: 581-589. PMid:16511590
View Article PubMed/NCBISchectman, G., S. Kaul, and A. H. Kissebah. 1989. Heterogeneity of low density lipoprotein responses to fish-oil supplementation in hypertriglyceridemic subjects. Arterioscler. Thromb. Vasc. Biol. 9: 345-354.
View ArticleShoelson, S. E., J. Lee, and A. B. Goldfine. 2006. Inflammation and insulin resistance. J. Clin. Invest. 116: 1793-1801. PMid:16823477
View Article PubMed/NCBIvan Hoek, M., J. G. Langendonk, S. R. de Rooij, E. J. G. Sijbrands, and T. J. Roseboom. 2009. Genetic variant in the IGF2BP2 gene may interact with fetal malnutrition to affect glucose metabolism. Diabetes 58:1440-1444. PMid:19258437
View Article PubMed/NCBIWallace, T. M., J. C. Levy and D. R. Matthews. 2004. Use and Abuse of HOMA Modeling. Diabetes Care. 27:1487–1495. PMid:15161807
View Article PubMed/NCBIWang, L., B. Waltenberger, E. M. Pferschy-Wenzig, M. Blunder, X. Liu, C. Malainer, T. Blazevic, S. Schwaiger, J. M. Rollinger, E. H. Heiss, D. Schuster, B. Kopp, R. Bauer, H. Stuppner, V. M. Dirsch and A. G. Atanasov. 2014. Natural product agonists of peroxisome proliferator-activated receptor gamma (PPARγ): a review Biochem. Pharmacol. 92: 73-89. PMid:25083916
View Article PubMed/NCBIWellen, K. E., and G. S. Hotamisligil. 2005. Inflammation, stress, and diabetes. J. lin. Invest. 115: 1111-1119. PMid:15864338
View Article PubMed/NCBIYamauchi, T., J. Kamon, H. Waki, Y. Terauchi, N. Kubota, K. Hara, Y. Mori, T. Ide, K. Murakami, N. Tsuboyama-Kasaoka, O. Ezaki, Y. Akanuma, O. Gavrilova, C. Vinson, M. L. Reitman, H. Kagechika, K. Shudo, M. Yoda, Y. Nakano, K. Tobe, R. Nagai, S. Kimura, M. Tomita, P. Froguel, and T. Kadowaki. 2001. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 7: 941-946. PMid:11479627
View Article PubMed/NCBIYang, Z., and Z.Q. Liu. 2008. Role of PPARγ in adipocyte differentiation and glucose and lipid metabolism. Int. J. Pathol. Clin. Res. 28:14-18.
Ye, J., Z. Gao, J. Yin, and Q. He. 2007. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am. J. Physiol. Endocrinol. Metab. 293: E1118-1128. PMid:17666485
View Article PubMed/NCBIYoon, M. J., G. Y. Lee, J. J. Chung, Y. H. Ahn, S. H. Hong, and J. B. Kim. 2006. Adiponectin increases fatty acid oxidation in skeletal muscle cells by sequential activation of AMP-activated protein kinase, p38 mitogen-activated protein kinase, and peroxisome proliferator-activated receptor alpha. Diabetes 55:2562-2570. PMid:16936205
View Article PubMed/NCBIYoshitomi, H., X. Guo, T. Liu, and M. Gao. 2012a. Guava leaf extracts alleviate fatty liver via expression of adiponectin receptors in SHRSP.Z-Leprfa/Izm rats. Nutr. Metab. (Lond) 9: 13. PMid:22348333
View Article PubMed/NCBIYoshitomi, H., L. L. Qin, T. H. Liu, M. Gao. 2012b. Guava Leaf Extracts Inhibit 3T3- L1 Adipocyte Differentiation Via Activating AMPK. J. Nut. Ther. 1: 107-113.
