Qunshan Wei
Tel.: +86 18017344008; Fax: +86 02167792557;
E-mail: qswei@dhu.edu.cn
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 5 ISSUE: 2
Page No: 51-60
Qunshan Wei
Tel.: +86 18017344008; Fax: +86 02167792557;
E-mail: qswei@dhu.edu.cn
Felix O. Mcyotto1, Qunshan Wei*, 1, 2, Christopher W.K. Chow3, Zuhair Nadeem1, Zheng Li 1, 2, Jianshe Liu 1, 2
1 School of Environmental Science and Engineering, Donghua University, Shanghai 201620, China.
2 Textile Pollution Controlling Engineering Center of Ministry of Environmental Protection, Shanghai 201620, China.
3 Natural and Built Environments Research Centre, School of Natural and Built Environments, University of South Australia, SA 5095, Australia.
Felix O. Mcyotto, Qunshan Wei, Christopher W.K. Chow, Zuhair Nadeem, Zheng Li, Jianshe Liu , Eco-friendly decolorization of cationic dyes by coagulation using natural coagulant Bentonite and biodegradable flocculant Sodium Alginate(2020) Journal of Earth Sciences & Environmental Studies 5(2) pp:51-60
The coagulation performance of Bentonite (BE) aided by Sodium Alginate (SA) to remove basic dyes was investigated. Four basic dyes Rhodamine B (RB), Malachite Green (MG), Methylene Blue (MB) and Basic Violet 14 (BV) were used to cover a range of variations in the experiments. It was found that the removal of the dyes increased with addition of SA as the flocculant/coagulant aid. Bentonite as a natural coagulant aided by the biodegradable flocculant, SA, is an effective combination for removal of basic dyes (color removal). The effect of coagulant dosage on the overall dye removal efficiency/decolorization rate was explored and followed by the investigation of the mechanism of dye removal by coagulation process. Optimum dye removal efficiencies for RB, MG, MB and BV by using both Bentonite and Sodium Alginate combined were, 91.5%, 98.2%, 98.5%, 98.8%, respectively with pH set at 9.0. FTIR was used to characterize the sludge to determine the functional groups. The resultant outcome in this study indicates that using Bentonite as a natural coagulant, aided by a biodegradable flocculant, Sodium Alginate, provides an effective and environmentally friendly coagulation option for removing color from basic dyes.
Keywords: dye removal efficiency; eco-friendly; coagulant dosage; biodegradable flocculant; natural coagulant
The world’s future and its biodiversity has become a general concern as it affects everyone. The development of industries and rapid technological advancement have led to the rise of numerous environmental problems especially pollution of water sources [1]. Different kinds of synthetic / man-made dyes end up in the wastewater discharges from various industries such as, textile, leather etc. These effluents are highly colored thus leading to serious pollution issues of the water bodies. The key contaminant to be easily identified in wastewater is usually color, owing to the visibility of synthetic dyes in aqueous solutions, even in very small quantities (<1.0 mgL-1). Dyes can be classified broadly as non-ionic, anionic or cationic based on the dye molecules’ ionic charge [2]. Cationic (basic) dyes have been reported to be highly toxic and carcinogenic compared with the anionic dyes [3]. The carcinogenic and toxicity nature of the basic dyes has made them a serious environmental concern; thus, it is necessary to ensure their removal before discharging to the environment.
However, the complexity of their aromatic structures and their synthetic origin makes their removal a daunting task; they are also biologically non-degradable which adds additional challenges to the wastewater treatment process [2].
Basic Violet 14 and Malachite Green dyes belong to the tri-phenyl methane dyes group which are normally containing compounds that are intensely colored. They have three aryl groups with each of the aryl groups being bonded to an atom of nitrogen; the nitrogen atoms interact either with one or two of the methyl groups [1]. It has been reported that Basic violet 14 can be potentially absorbed through human skin and has cytotoxicity on human red blood cells. Malachite green dye when ingested is toxic, carcinogenic and hazardous [4]. It affects humans, mice and fish by leading to kidney and liver tumors. It also causes their reproductive systems to malfunction and increase probability of cancer of the bladder [5].
Methylene blue dye belongs to the thiazine group of basic dyes. It can be either in green crystalline or powder form and is the most vital basic dye [6]. Methylene Blue has been reported to be harmful to living organisms including for short exposure period [2]. Rhodamine B is reported to be highly soluble in water and belongs to the xanthene class [7]. It harms human beings and animals when swallowed and also irritates the respiratory tract, eyes and the skin [8]. Its carcinogenicity, neurotoxicity, reproductive and developmental toxicity, and chronic toxicity to animals and humans has been robustly proven through experiments [9–12]. The use of Rhodamine B dye as food colorant has been banned based on the above health concerns [13]. Because of these toxic and hazardous effects of basis dyes, it is necessary to remove them from any wastewater before discharging them to the environment.
There are several physio-chemical and biological methods used to treat wastewater with dye presence. The treatment methods include sorption [14,15], oxidation/ozonation [16–18], coagulation/flocculation [19–21], anaerobic/aerobic [22–24], photocatalytic degradation [21,25,26] and membrane separation [27,28]. Dyes are stable towards oxidizing agents and light and this complicates their removal through many different treatment steps. Hence, careful and thoughtful selection of the removal methods is critical and vital [29,30]. Several adsorption studies have been undertaken to treat basic dyes including using bentonite[2,6,31]. However, adsorption process poses the challenge of high chemical cost for absorbent use[32]. Therefore, coagulation has been considered as the most suitable option and was used in this studies to decolorize basic dyes due to its numerous advantages including low capital cost [33,34], ease of use, simplicity of design requirements, low energy consumption and high efficiency.
Synthetic polymers have been proven to improve the efficiency of filtration performance and aide strengthening of flocs which has been applied widely in improving the recoverability and floc strength. However, synthetic polymers have been reported to have potency for harming human health[35]. Cationic polyacrylamides, Polydiallyldimethylammoniumchloride and polyamines have been found to yield carcinogenic by-product N-nitroso dimethylamine (NDMA), during the ozonation process; polydiallyldimethylammonium chloride produces high concentration of NDMA in several water treatment plants. In view of the health and safety threats posed by synthetic polymers such as APAM, there is an increasing need for coagulant aid that are not only environmentally friendly, but also effective [36].
Raw bentonite is primarily a smectite nonmetallic clay mineral and is composed of two silica tetrahedral sheets that have a central Al octahedral sheet (2:1 type) [37]. The isomorphous substitution in the tetrahedral and octahedral layers, of the Al3+ for Si4+ and the Mg2+ for Al3+ respectively, gives rise to permanent negative charges on bentonite [38]. Its lattice structure has exchangeable cations (such as Cu2+, Mg2+, K+ Na+, Ca2+ etc.) that balance the negative charge. Its montmorillonite and cationic crystal cell are very unstable thus these cations can easily be replaced by other cations. Through ion exchange mechanism the organic cations are exchanged by its interlayer inorganic cations. When in aqueous solution the entering of water molecules into its lattice structure causes swelling of the clays. Due to this, it has been reported by other researchers that bentonite through cationic exchange gives good adsorbing performance for cationic pollutants [37,38]. Bentonite has been used in various studies as an adsorbent to remove color from wastewater. However, very small number of research studies have been done on its application in conjunction with sodium alginate in coagulation process for basic dyes, such as Rhodamine B, Malachite Green, Methylene Blue and Basic Violet 14.
Sodium Alginate is a sodium salt of the alginic acid. It is an anionic polymer that has linear chain structure and is water soluble [36]. It is eco-friendly and used widely in production of beads, microsphere, microcapsule and drug tablets [39]. Sodium Alginate has been used reportedly as a coagulant aid to enhance the removal of humic acid [36]. In principle it can improve flocs characteristics and overall coagulation efficiency. However, to the best of our current knowledge, it has not been used in treating basic dyes or improving their color removal rate efficiency using Bentonite. In this paper, the decolorization of basic dyes (Rhodamine B, Malachite Green, Methylene Blue and Basic Violet 14) from wastewater using Bentonite aided by Sodium Alginate was carried out. Sodium Alginate was used to investigate its potential to provide a natural substitute to synthetic polymers and also improve the performance of dye removal efficiency of Basic dyes using Bentonite.
2.1. Chemicals, dyes and coagulants
Four commercial dyes, one coagulant and one flocculant from the suppliers, were used in their original form. Bentonite (BE) and Sodium Alginate (SA) were of reagent grade and procured from Sinopharm Chemical Reagent Co. Ltd. The dyes were procured from commercial suppliers in China. pH adjustment was done using NaOH and HCl which were supplied by Sinopharm Chemical reagent Co. Ltd and were of reagent grade.
2.2. Preparation of dye solutions, Sodium Alginate and Bentonite
The dyes used in this study were Rhodamine B, Basic Violet 14, Malachite Green and Methylene Blue. Each dye was used to prepare its own test dye stock solution by adding 1g of the dye-stuff to 100ml of deionized water. Dye waste water samples of 10g/L concentration was then prepared from this suspension. 0.1% Sodium Alginate stock solution was prepared by dissolving 1g Sodium Alginate into 1000 ml deionized water and stirred using magnetic stirrer for 1 h. This sodium alginate stock solution was refrigerated at 4 degree Celsius. Bentonite was sieved using a 75 micrometers particle size sieve then 1% Bentonite stock solution prepared by dissolving 5g bentonite into 500ml of distilled water, and stirred using magnetic stirrer for an hour. This was then followed by sonication for 15 mins at 40 Hz. The bentonite stock solution was sonicated for 5 mins every time before use to achieve homogeneity.
2.3. Experimental Procedure
The four dye test solution samples were used to conduct laboratory experiments using predetermined coagulant and coagulant aid dosages. The dye test solutions were used to examine the dye removal efficiency of different basic dyes using Bentonite aided by Sodium Alginate; it was aimed that the use of pure dye test solutions would make it possible to obtain this information. Table 1 gives the names, chromophoric groups, the maximum absorbance wavelengths and the molecular weights of the four dyes used for this study, whereas Fig 1 provides their molecular structures.
The jar testing procedure was used to determine the optimum dosages of coagulants required for dye removal. The optimum pH value 9 was set as per previous research [40,41]. 200 ml of dye test solutions were poured in 250ml beakers, which were then placed on a six- beaker jar testing apparatus with paddles. A period of 1 min flash mixing at 300 rpm was allowed followed by slow mixing at 50 rpm for 10 min. When using the coagulant and coagulant aid together, the coagulant was first added followed by the addition of the coagulant aid after 10 seconds. 30 min was allowed for sedimentation. This was followed by pipetting of the supernatant aliquot and then filtration using a 0.45 um membrane (Mixed Cellulose Ester- MCE), prior to analyses. The dye removal efficiency using bentonite and sodium alginate was quantified using a UV-Vis spectrophotometer (analytikjena-SPECORD 200 PLUS). For each absorbance measurements, the maximum absorbance wavelength ( l max) of each dye was employed. Part of the sludge from the treated waste waters was filtered then dried and analyzed using FTIR meter (Brooktensor 27).
The percentages of dye removal efficiency were calculated by comparing the absorbance values before (original dye test solution) and after treatment (as shown below by Eq. 1).
Dye Removal Efficiency % = C0-Cf/C0 x 100% -Eq. 1
where, Co and Cf are the dye concentration in original and treated dye solutions respectively. Deionized water used as a reference.
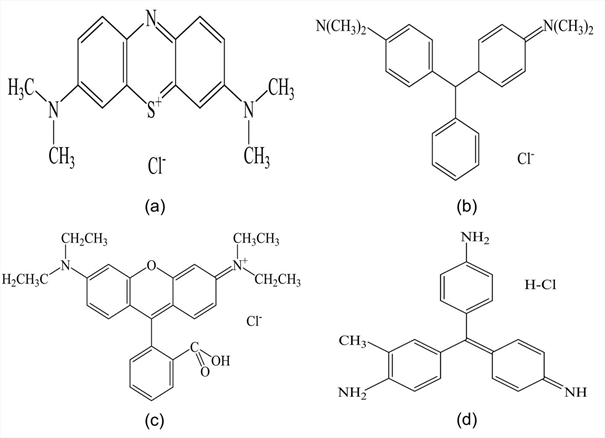
Fig. 1: Structures of basic dyes; a) Methylene Blue, b) Malachite Green, c) Rhodamine B, d) Basic Violet 14
Table 1. Dye Characteristics
Dye Characteristics – Chromophoric group, maximum absorbance wavelength and molecular weight
|
Name |
Chromophoric group |
Wavelength absorbed (nm) |
Molecular Weight (g/mol) |
|
Methylene Blue (MB) |
Thiazine |
598 |
319.9 |
|
Basic violet 14 (BV) |
Triaryl methane |
534 |
337.85 |
|
Rhodamine B (RB) |
xanthene |
524 |
380.82 |
|
Malachite Green (MG) |
Triaryl methane |
618 |
364.9 |
3.1. Effect of coagulant dosage on dye color removal of Rhodamine B, Malachite Green, Methylene Blue and Basic Violet 14
For this study Bentonite in the range of 0-200mg/L and SA 0-20mg/L were used to assess their individual standalone performance, in removal of four basic dyes (Rhodamine B, Basic violet 14, Methylene Blue and Malachite Green). Temperature, mixing time and other critical coagulation variables were kept constant, enabling for comparison, coagulant dosage to be set as single variable. Fig 2 shows the results.
For Rhodamine B dye the highest dye removal efficiency by Bentonite (as sole coagulant) and Sodium alginate (as sole coagulant) at dosages of 200mg/L and 20mg/L were 53.7 % and 1.8 %, respectively. Bentonite achieved the highest dye removal efficiency of 53.7% for Rhodamine B dye (Fig. 2a). Increase in the dosage of bentonite led to gradual increase in the dye removal efficiency until it reached the plateau. For instance, at bentonite dosages of 40 mg/L, 120 mg/L and 160 mg/L the dye removal efficiencies were 3.4 %, 16.3 % and 42.8%, respectively. Sodium Alginate exhibited lower dye removal efficiency for RB dye, attaining its highest dye removal efficiency of only 1.8 % at a dosage of 20 mg/L (Fig. 2b). Gradual increment in dosage of Sodium Alginate also led to the increase in dye removal efficiencies of RB dye. Sodium Alginate dosages of 4 mg/L,12 mg/L and 16 mg/L attained dye removal efficiencies of 0.9 %, 1.3 % and 1.5 %, respectively.
For Malachite Green dye the highest dye removal efficiencies by Bentonite (as sole coagulant) and Sodium alginate (as sole coagulant) at dosages of 160mg/L and 20mg/L were 97.9 % and 75.5 % respectively. Bentonite achieved the highest removal efficiency of 97.9 % for MG dye. Increase in the dosage of bentonite led to gradual increase in the dye removal efficiency. For instance, at bentonite dosages of 40 mg/L, 120 mg/L and 160 mg/L the dye removal efficiencies were 97.2 %, 97.9 % and 97.9 % respectively. Sodium Alginate on the other hand achieved dye removal efficiency of 75.5 % at a dosage of 20mg/L. Gradual increment in dosage of Sodium Alginate also led to the increase in dye removal efficiencies of MG. Sodium Alginate dosages of 4 mg/L,12 mg/L and 16 mg/L attained dye removal efficiencies of 34.5%, 66.9 % and 71.2 % respectively.
For Basic Violet 14 dye the highest dye removal efficiencies by Bentonite (as sole coagulant) and Sodium alginate (as sole coagulant) of 200mg/L and 8mg/L were 97.6 % and 56.6 % respectively. Bentonite achieved the highest removal efficiency of 97.5 % for BV dye.
Increase in the dosage of bentonite led to gradual increase in the dye removal efficiency. For instance, at bentonite dosages of 40 mg/L, 120 mg/L and 160 mg/L the dye removal efficiencies were 63.6 %, 95.9 % and 97.2 % respectively. Sodium Alginate achieved on the other hand, dye removal efficiency of 56.6 % at a dosage of 8mg/L. Increasing the dosage of Sodium Alginate also led to the increase in dye removal efficiency until the highest value was attained, after which further increment of dosage led to decrease in dye removal efficiency. Sodium Alginate dosages of 4 mg/L,12 mg/L and 16 mg/L attained dye removal efficiencies of 32.8 %, 33.6 % and 35.2 % respectively.
For Methylene Blue dye the highest dye removal efficiency by Bentonite (as sole coagulant) and Sodium alginate (as sole coagulant) at dosages of 200mg/L and 20mg/L were 97.2 % and 81.5 %, respectively. Bentonite achieved the highest dye removal efficiency of 99.0 % for MB dye. Increase in the dosage of Bentonite and Sodium Alginate led to gradual increase in the dye removal efficiency. For instance, at bentonite dosages of 40 mg/L, 120 mg/L and 160 mg/L the dye removal efficiencies were 4.5 %, 80.2 % and 97.0 %, respectively. While, Sodium Alginate achieved dye removal efficiency of 81.5 % at a dosage of 20mg/L. In addition, Sodium Alginate dosages of 4 mg/L,8 mg/L and 12 mg/L attained dye removal efficiencies of 31.0 %, 78.0 % and 81.0 %, respectively.
The above results indicate that the best dye removal efficiency for all the four basic dyes was achieved by Bentonite. Increment in dosage of Bentonite (BE) and Sodium Alginate (SA) resulted in increase in dye color removal rate. Basic dyes are positively charged in aqueous solution, thus, addition of BE and SA which are anionic leads to occurrence of charge neutralization. In addition, this results in driving of the cations from the basic dyes onto the negatively charged surface of Bentonite, that is adsorption bridging mechanism occurs[38].
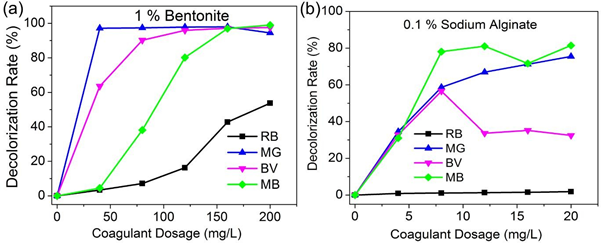
Fig. 2: Effect of coagulant dosage on the efficiency of color removal of basic dyes; a) Bentonite, b) Sodium Alginate
3.2 Effect of Sodium alginate as coagulant aid on basic dyes removal efficiency using Bentonite
In this study, BE was selected as coagulant and SA as a coagulant aid; the removal performance of this combination was assessed. Combinations of BE and SA (4mg/L and 12mg/L) were used as shown in Fig. 3. The highest dye removal efficiency for Rhodamine B dye was 91.5 % at a combined dosage of 160 mg/L Bentonite and 4 mg/L Sodium Alginate. The highest dye removal efficiency for Basic violet 14 dye was 98.8 % at a combined dosage of 160 mg/L Bentonite and 4 mg/L Sodium Alginate. The highest color removal rate for Methylene blue dye was 98.5 % at a combined dosage of 160 mg/L Bentonite and 160 mg/L Sodium Alginate. Finally, the highest dye removal efficiency for basic green 4 dye was 98.2% at a combined dosage of 160 mg/L Bentonite and 160 mg/L Sodium Alginate.
A combination of BE and SA for the two dosages of SA yielded an increment in color removal rate for RB, MB, MG and BV dyes. Bentonite particles are reported to have on their surface very large negative charge [42] thus its ability to achieve very high color removal rates for most basic dyes. The addition of Sodium Alginate which is also anionic enhances the removal of basic dyes through coagulation/flocculation process using natural coagulant Bentonite. As indicated by these results, a combination of Bentonite and Sodium Alginate is highly effective in basic dyes color removal by coagulation, even for the Rhodamine B which is very problematic to treat.
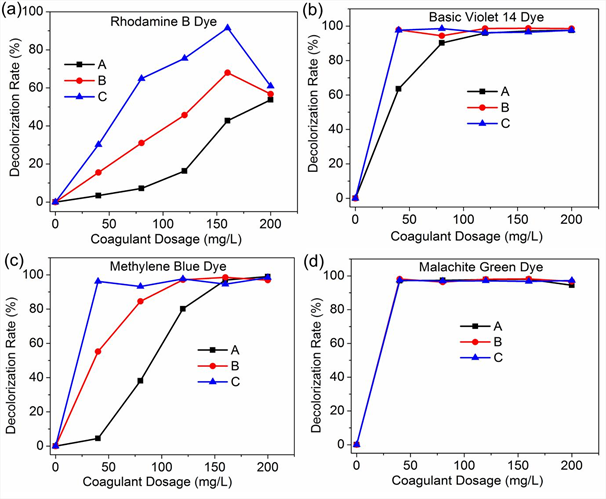
A - Bentonite
B - Bentonite + Sodium Alginate (4 mg/L)
C - Bentonite + Sodium Alginate (12mg/L)
Fig. 3: Effect of Sodium alginate as coagulant aid on basic dyes color removal using Bentonite; a) Rhodamine B dye, b) Basic Violet 14 dye, c) Methylene Blue dye, d) Malachite Green dye
3.3. Effect of zeta potential change on dye color removal efficiency by coagulation
MG-bentonite achieved the highest dye removal efficiency at a lower dosage than the other basic dyes, thus was chosen for zeta potential measurements; results are shown in Fig 4. An increase in bentonite dosage resulted in the rise of MG dye removal efficiency (Fig 4a) and zeta potential (Fig. 4b). MG-bentonite combination for the applied Bentonite dosages had zeta potential between -13.83 mv and – 32.07 mv. With increase in coagulant dosage, the zeta potential of MG decreased owing to its cationic nature, implying charge neutralization occurred. It has been reported that colloids exhibit moderate stability at a zeta potential range of ± 30 to ± 40 ,whereas exhibit incipient instability between 10 to ± 30[43] . The zeta potential of MG-BE for various bentonite dosages was not close to zero implying that charge neutralization didn’t play a major role in MG color removal and also wasn’t the sole coagulation mechanism[44].
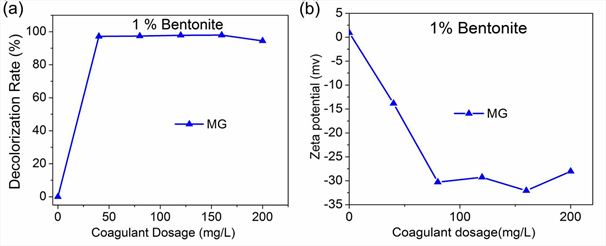
Fig. 4: The effect of Bentonite dosage on MG dye removal efficiency and Zeta potential; a) Dye removal efficiency, b) Zeta potential
3.4. Analysis of coagulation precipitates by FTIR
MG was chosen for this experiment as it achieved the highest dye removal efficiency at the lowest dosage than the other basic dyes. The FTIR test was employed so as to clarify further the internal mechanism of the combination of bentonite and the basic dyes. FTIR meter (Brooktensor 27) was used to analyze dried sludges resulting from the treatment of the dye test water. The FTIR analysis results are shown in Fig 5.
The sharp adsorption peak at 1585 cm-1 is due to the benzene ring C=C stretching, whereas, that at 1375 cm-1 is assigned to –CH3 stretching in the malachite green[45]. The several FTIR spectra peaks at the MG fingerprint region of 400-800 cm-1 represent mono- substituted and para-distributed rings of benzene[45].The FTIR spectra band of bentonite at 3441 cm-1 denotes H2O group stretching vibrations, 1035 cm-1 denotes Si-O stretching while 529 cm-1 and 462 cm-1 denote Si-O, Si-O-Si and Al-O-Si deformations[46]. The pure bentonite and MG-bentonite patterns at this region appear basically the same, that is, essentially unchanged. Moreover, the bentonite and MG-bentonite spectra patterns also appear very similar which suggested that the MG dye has been adsorbed onto the bentonite and no new chemical species has been formed.
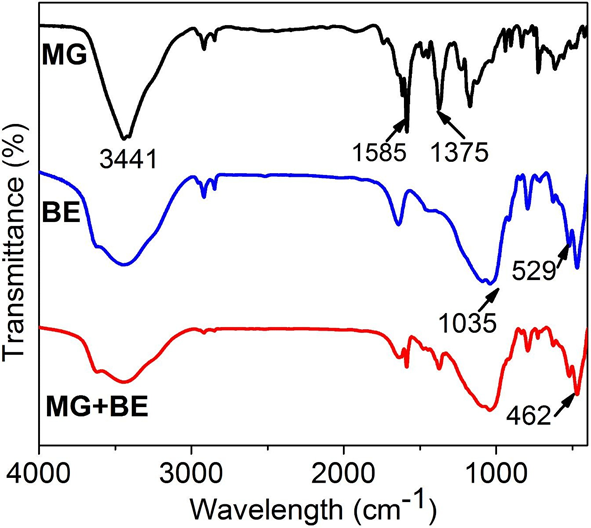
Fig. 5: FTIR spectra of MG, BE and MG+BE
3.5. Mechanism of color removal by coagulation
Dissolution of MG in water released positive charges owing to its cationic nature as shown Fig 4. Bentonite on the other hand is anionic thus was able to induce charge neutralization thereby removing MG color through coagulation. Increment in bentonite dosages led to increase in dye removal efficiency as zeta potential reduction occurred. However, the MG-bentonite system even at lowest dosages of bentonite (40mg/L) had a negative zeta potential implying that all the cations from MG had been neutralized. This suggests that charge neutralization was not the only mechanism at play. FTIR analysis revealed adsorption of MG onto bentonite as shown by Fig 5. The presence of –CH3, -– N(CH3 )2 and C=C cause bridging adsorption to occurred alongside charge neutralization as shown by Fig 5: the MG+BE FTIR spectra in the regions denoting them is totally similar to that of BE.
From this study, the key to effective dye removal of basic dyes by coagulation / flocculation method is the use of anionic coagulants and coagulant aids with good adsorptive ability, to boost charge neutralization and bridging adsorption, with bridging adsorption being the most important as shown by Fig 4 and 5. The combination of Bentonite as coagulant and Sodium alginate as coagulant aid results in improvement of dye removal efficiency of basic dyes. Bentonite on its own yields high dye removal efficiency for tri-phenyl methane (BV & MG) and thiazine group (MB) basic dyes, but achieves considerably lower removal efficiency for xanthene (RB) group basic dyes.
The combination of bentonite with Sodium Alginate improves the dye removal efficiency of basic dyes. For instance, RB which had a lower dye removal efficiency of 53.7% when treated with bentonite alone, but exhibited an improved dye removal efficiency of 91.5% when BE was combined with SA. A combination of bentonite and sodium alginate provides an effective and eco-friendly option for removing dye color through coagulation/flocculation process, from highly toxic basic dye waste waters.
This work was supported by the National Key Research and Development Program of China (Grant No. 2016YFC0400501, 2016YFC0400509), the National Natural Science Foundation of China (NSFC)( No. 21876025 ) and the National water pollution control key project (2017ZX07202005-005).
Bonetto LR, Ferrarini F, De Marco C, et al. Removal of methyl violet 2B dye from aqueous solution using a magnetic composite as an adsorbent. J. Water Process Eng. 2015;6:11-20.
View ArticleEl-Sayed GO. Removal of methylene blue and crystal violet from aqueous solutions by palm kernel fiber. Desalination. 2011;272:225-232.
View ArticleHao OJ, Kim H, Chiang PC. Decolorization of wastewater. Crit. Rev. Environ. Sci.Technol. CRC Press LLC; 2000. p. 449-505.
View ArticleGründker C, Völker P, Emons G. Antiproliferative Signaling of Luteinizing Hormone- Releasing Hormone in Human Endometrial and Ovarian Cancer Cells through G Proteinα I -Mediated Activation of Phosphotyrosine Phosphatase 1. Endocrinology [Internet]. 2001 [cited 2019 Nov 19];142:2369-2380. PMid:11356684
View Article PubMed/NCBIGautam RK, Rawat V, Banerjee S, et al. Synthesis of bimetallic Fe-Zn nanoparticles and its application towards adsorptive removal of carcinogenic dye malachite green and Congo red in water. J. Mol. Liq. [Internet]. 2015 [cited 2019 Nov 20];212:227-236.
View ArticleHan R, Zou W, Yu W, et al. Biosorption of methylene blue from aqueous solution by fallen phoenix tree's leaves. J. Hazard. Mater. 2007;141:156-162. PMid:16901629
View Article PubMed/NCBIJain R, Mathur M, Sikarwar S, et al. Removal of the hazardous dye rhodamine B through photocatalytic and adsorption treatments. J. Environ. Manage. 2007;85:956- 964. PMid:17239520
View Article PubMed/NCBIRochat J, Demenge P, Rerat JC. [Toxicologic study of a fluorescent tracer: rhodamine B]. Toxicol. Eur. Res. [Internet]. 1978 [cited 2019 Nov 19];1:23-26.
View ArticleShimada T, Yamazaki H, Mimura M, et al. Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: Studies with liver microsomes of 30 Japanese and 30 Caucasians [Internet]. J. Pharmacol. Exp. Ther. 1994 [cited 2019 Nov 19]. p. 414-423.
View ArticleOpinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) to review the toxicology of a number of dyes illegally present in food in the EU. EFSA J. [Internet]. 2005 [cited 2019 Nov 19];3:263.
View ArticleKornbrust D, Barfknecht T. Testing of 24 food, drug, cosmetic, and fabric dyes in the in vitro and the in vivo/in vitro rat hepatocyte primary culture DNA repair assays. Environ. Mutagen. [Internet]. 1985 [cited 2019 Nov 19];7:101-120. PMid:3967633
View Article PubMed/NCBIMcGregor DB, Brown AG, Howgate S, et al. Responses of the L5178Y mouse lymphoma cell forward mutation assay. V: 27 coded chemicals. Environ. Mol. Mutagen. [Internet]. 1991 [cited 2019 Nov 19];17:196-219. PMid:1902415
View Article PubMed/NCBIGu VK, Merck E. Re m o v a l o f Rh o d a m in e B , F a s t Gre e n , a n d Me th y le n e B lu e fro m Wa s te w a te r U s in g Re d Mu d , a n Alu m in u m In d u s try Wa s te. 2004;1740-1747
Bhatnagar A, Sillanpää M. Utilization of agro-industrial and municipal waste materials as potential adsorbents for water treatment-A review. Chem. Eng. J. 2010. p. 277-296
View ArticleFu L, Shuang C, Liu F, et al. Rapid removal of copper with magnetic poly-acrylic weak acid resin: Quantitative role of bead radius on ion exchange. J. Hazard. Mater. [Internet]. 2014 [cited 2019 May 23];272:102-111. PMid:24681592
View Article PubMed/NCBIMalik PK, Saha SK. Oxidation of direct dyes with hydrogen peroxide using ferrous ion as catalyst. Sep. Purif. Technol. 2003;31:241-250. 00200-9
View ArticleKim T-H, Park C, Shin E-B, et al. Effects of Cl-based chemical coagulants on electrochemical oxidation of textile wastewater. Desalination [Internet]. 2003 [cited 2019 Sep 3];155:59-65. 00239-X
View ArticleCiorba G, Radovan C, Vlaicu I, et al. Colour removal from simulated dye wastewaters by electrochemical treatment. dnph.phys.msu.ru [Internet]. [cited 2019 Sep 3];
Panswad T, Wongchaisuwan S. Mechanisms of dye wastewater colour removal by magnesium carbonate-hydrated basic. Water Sci. Technol. 1986;18:139-144.
View ArticleOzkan A, Yekeler M. Coagulation and flocculation characteristics of celestite with different inorganic salts and polymers. Chem. Eng. Process. Process Intensif. [Internet]. 2004 [cited 2019 May 8];43:873-879. 00107-7
View ArticleAli H, Chaitanya K, Professor A. A Review on Effluent Treatment of Textile by Biological and Chemical Methods [Internet]. 2017 [cited 2019 Nov 12].
Chinwetkitvanich S, Tuntoolvest M, Panswad T. Anaerobic decolorization of reactive dyebath effluents by a two-stage UASB system with tapioca as a co-substrate. Water Res. 2000;34:2223-2232. 00403-0
View ArticleTaştan BE, Karatay SE, Dönmez G. Bioremoval of textile dyes with different chemical structures by Aspergillus versicolor in molasses medium. Water Sci. Technol. [Internet]. 2012 [cited 2019 May 23];66:2177-2184. PMid:22949249
View Article PubMed/NCBIKapdan IK, Alparslan S. Application of anaerobic-aerobic sequential treatment system to real textile wastewater for color and COD removal. Enzyme Microb. Technol. [Internet]. 2005 [cited 2019 May 23];36:273-279.
View ArticlePeik-See T, Pandikumar A, Ngee LH, et al. Magnetically separable reduced graphene oxide/iron oxide nanocomposite materials for environmental remediation. Catal. Sci. Technol. [Internet]. 2014 [cited 2019 May 23];4:4396-4405.
View Articledos Santos AB, Cervantes FJ, van Lier JB. Review paper on current technologies for decolourisation of textile wastewaters: Perspectives for anaerobic biotechnology. Bioresour. Technol. 2007. p. 2369-2385 PMid:17204423
View Article PubMed/NCBICiardelli G, Corsi L, Marcucci M. Membrane separation for wastewater reuse in the textile industry. Resour. Conserv. Recycl. 2001;31:189-197 00079-3
View ArticleWei X, Kong X, Wang S, et al. Removal of Heavy Metals from Electroplating Wastewater by Thin-Film Composite Nanofiltration Hollow-Fiber Membranes. Ind. Eng. Chem. Res. [Internet]. 2013 [cited 2019 May 23];52:17583-17590.
View ArticleLambert SD, Graham NJD. Adsorption methods for treating organically coloured upland waters. Environ. Technol. Lett. 1989;10:785-798.
View ArticleGupta VK, Mittal A, Krishnan L, et al. Adsorption kinetics and column operations for the removal and recovery of malachite green from wastewater using bottom ash. Sep. Purif. Technol. 2004;40:87-96.
View ArticleRai P, Gautam RK, Banerjee S, et al. Synthesis and characterization of a novel SnFe
Ahmad A, Mohd-Setapar SH, Chuong CS, et al. Recent advances in new generation dye removal technologies: Novel search for approaches to reprocess wastewater. RSC Adv. Royal Society of Chemistry; 2015. p. 30801-30818.
View ArticleGolob V, Vinder A, Simonič M. Efficiency of the coagulation/flocculation method for the treatment of dyebath effluents. Dye. Pigment. 2005;67:93-97.
View ArticleAnjaneyulu Y, Sreedhara Chary N, Samuel Suman Raj D. Decolourization of Industrial Effluents - Available Methods and Emerging Technologies - A Review. Rev. Environ. Sci. Bio/Technology [Internet]. 2005 [cited 2019 May 8];4:245-273.
View ArticleOladoja NA, Aliu YD. Snail shell as coagulant aid in the alum precipitation of malachite green from aqua system. J. Hazard. Mater. [Internet]. 2009 [cited 2019 Nov 20];164:1496-1502. PMid:19013019
View Article PubMed/NCBIWang Y, Li X, Wu C, et al. The role of sodium alginate in improving floc size and strength and the subsequent effects on ultrafiltration membrane fouling. Environ. Technol. (United Kingdom). 2014;35:10-17. PMid:24600835
View Article PubMed/NCBIHuang Z, Li Y, Chen W, et al. Modified bentonite adsorption of organic pollutants of dye wastewater. Mater. Chem. Phys. [Internet]. 2017;202:266-276.
View ArticleAnirudhan TS, Ramachandran M. Adsorptive removal of basic dyes from aqueous solutions by surfactant modified bentonite clay (organoclay): Kinetic and competitive adsorption isotherm. Process Saf. Envion. Prot. [Internet]. 2015 [cited 2019 Nov 20];95:215-225.
View ArticleKaneko K, Kanada K, Miyagi M, et al. Formation of water-insoluble gel in dry-coated tablets for the controlled release of theophylline. Chem. Pharm. Bull. [Internet]. 1998 [cited 2019 Nov 19];46:728-729. PMid:9579051
View Article PubMed/NCBIVerma AK, Dash RR, Bhunia P. A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. J. Environ. Manage. [Internet]. 2012 [cited 2019 May 8];93:154-168. PMid:22054582
View Article PubMed/NCBIAnirudhan TS, Ramachandran M. Adsorptive removal of basic dyes from aqueous solutions by surfactant modified bentonite clay (organoclay): Kinetic and competitive adsorption isotherm. Process Saf. Environ. Prot. [Internet]. 2015;95:215-225.
View Article張元月, 中村文雄, 坂本康, et al. Coagulation Behaviors and Coagulant Dosage Control of Kaolin and Bentonite Suspensions. Environ. Eng. Res. 2002;39:1-7.
Sharma AK, Priya, Kaith BS, et al. Selective removal of cationic dyes using response surface methodology optimized gum acacia-sodium alginate blended superadsorbent. Int. J. Biol. Macromol. [Internet]. 2019;124:331-345. PMid:30481534
View Article PubMed/NCBIYue QY, Gao BY, Wang Y, et al. Synthesis of polyamine flocculants and their potential use in treating dye wastewater. J. Hazard. Mater. 2008;152:221-227. PMid:17689006
View Article PubMed/NCBIJiang F, Dinh DM, Hsieh Y Lo. Adsorption and desorption of cationic malachite green dye on cellulose nanofibril aerogels. Carbohydr. Polym. [Internet]. 2017;173:286-294. PMid:28732868
View Article PubMed/NCBIMekhamer WK. The colloidal stability of raw bentonite deformed mechanically by ultrasound. J. Saudi Chem. Soc. [Internet]. 2010;14:301-306.
View Article