Changlian Peng
Email: pengchl@scib.ac.cn
© 2019 Sift Desk Journals. All Rights Reserved
Changlian Peng
Email: pengchl@scib.ac.cn
Qilei Zhang1, Zhengchao Yu1, Minling Cai, Guangxin Chen, Changlian Peng*
Guangdong Provincial Key Laboratory of Biotechnology for Plant Development,School of Life Sciences, South China Normal University, Guangzhou 510631, China
J Simal-G%C3%A1ndara(jsimal@uvigo.es)
Raffaele Capasso(rafcapas@unina.it)
Toni J Lopes(tonijl@unochapeco.edu.br)
Qilei Zhang, Zhengchao Yu, Minling Cai, Guangxin Chen, Changlian Peng, Purification and antioxidative function of anthocyanins from fructus rhodomyrti(2019) Journal of Plant Science 3(2) p:167-173
Background: Fructus rhodomyrti, as a colorful fruit, is rich in anthocyanins and anthocyanins have strong antioxidant capacity.
Methods: The purification and antioxidative capacity of anthocyanins from Fructus rhodomyrti (fruit of downy rose myrtle, AFR) were studied and compared to those of black rice (ABR).
Results: The result of the thiobarbituric acid reactive substances (TBARS) assay showed that both AFR and ABR effectively prevented the oxidation of thiobarbituric acid and reduced the production of malondialdehyde (MDA). The restriction effect of AFR was slightly weaker than that of ABR, but no significant difference was observed. The antioxidative activities of AFR toward H2O2 and epinephrine were also slightly weaker than those of ABR.
Conclusion: Fructus rhodomyrti, which has an abundance of anthocyanins and antioxidation activities comparable to those of black rice, has excellent potential as a health care product and should be further explored. However, considering the ecological restoration function of downy rose myrtle, the use of anthocyanins from black rice was suggested as being more economical, more ecological and more effective.
Key words: Anthocyanins; Antioxidative activity; Downy rose myrtle; Macroporous resins; Radical scavenging activity
It has been widely reported that reactive oxygen species (ROS) play an important role in lifestyle- and age- related diseases, such as diabetes mellitus, atherosclerosis and tumors [1]. Accompanying with the development of self-care awareness, dietary intake of antioxidants is considered an important way to prevent these pathophysiological conditions [2]. Most previous research has focused on the antioxidant component in food resources, such as lignans in common Sinopodophyllum fruit [3], flavonoids in onions [4], phenolic compounds in strawberry [5], proanthocyanidins in red wine Pine bark [6], and catechins in tea [7]. Previous studies have showed that the phenol extracted from virgin olive oils had the function of neuroprotection and antidiabetic [8,9], and the phenol was cytotoxic to breast cancer cells [10], the phenol extracted from pistachio kernel could inhibit the proliferation of breast cancer cells [11], polyphenol-protein interactions can be used for improving food quality [12]. As an important group of antioxidative pigments, anthocyanins also receive a great deal of attention. To date, there are 17 known naturally occurring anthocyanins or aglycones widely spread in flowers, fruits and leaves, of which 6 anthocyanins are common in higher plants [13]. Based on their antioxidant properties, plants or fruits with abundant anthocyanins have usually been used by humans for therapeutic purposes [14].
Fructus rhodomyrti is the fruit of Rhodomyrtus tomentosa (Ait.) Hassk and is usually a bright purplish-red color due to the presence of anthocyanins. The average size of fructus rhodomyrti is 52.68 mm×11.76 mm (long×width), and the average fresh weight is 1.39 g [15]. Fructus rhodomyrti is usually used as a traditional Chinese medicine to nourish the blood and relieve diarrhea [16]. It has been demonstrated that there are 18 amino acids and an abundance of minerals and vitamins in fructus rhodomyrti [17,18]. Strawberry tree honey was considered as a new functional food because it contained a lot of phenolic ketones and amino acids, and had anti-cancer effect [19,20]. The extract of fructus rhodomyrti also has strong antioxidant activities for the major component anthocyanins [21]. In recent years, increasing attention has focused on the exploitation of fructus rhodomyrti. Some fructus rhodomyrti beverages have been explored for health care purposes [22]. However, research on the purification and antioxidative capacity of fructus rhodomyrti anthocyanins is still rare. Study showed that food colorant could be extracted and purified from colorful fruits [23,24]. In this study, we improved the purification method of fructus rhodomyrti anthocyanins and evaluated their antioxidant activities in contrast to those of black rice. The contribution of fructus rhodomyrti to dietary intake of anthocyanins was also discussed.
2.1. Materials
Fructus rhodomyrti was obtained from the South China Botanical Garden, Chinese Academy of Sciences, subtropical China. For each cultivar, 250-300 g fully of mature fructus rhodomyrti was collected and stored at 4°C until further treatment. After washing and weighing, fructus rhodomyrti was mixed in an electric blender and samples were extracted with a 500 ml mixture of MeOH and 0.1 mol/l HCl (MeOH:HCl=85:15, v/v) [25]. The extracts were then centrifuged at 12000 g for 15 min. The supernatants were collected and stored at -20°C. Black rice was purchased from the PARKNSHOP supermarket, Tianhe, Guangzhou. It was extracted and treated in the same way as fructus rhodomyrti.
2.2. Measurements
Five resins, i.e., D4020, D-3520, NKA-9, AB-8 and D101A (Chemical Industrial Company, affiliated with Shanghai Jiaotong University, Shanghai, China, Table 1), were tested to determine the best resins for capturing anthocyanins from AFR. All the resins were cross-linked polystyrene copolymers. The resins were pretreated and activated according to the manufacturer’s recommendations. First, the five resins were rinsed with distilled water and filtered with nylon filter cloth (mesh size=0.3 mm) and soaked overnight in 2 bed volumes (BV) of 95% ethanol. After soaking, the resins were placed into a glass column and further rinsed with 2 BV of 95% ethanol. Subsequently the glass column was orderly rinsed with 2 BV of distilled water, 1 BV of 4% (w/v) sodium hydroxide, 2 BV of distilled water, 1 BV 4% (v/v) hydrochloric acid, and distilled water until pH =7.4 was obtained.
The AFR extract was passed through AB-8 resin columns. Anthocyanins and other phenolics were adsorbed onto the resins; sugar, acids, and other water-soluble compounds were eluted with more than 2 BV of distilled water until the wash water was clear. The adsorbed material was then eluted with acidified ethanol until there was no color in the eluent. The eluent was concentrated on a rotary evaporator under reduced pressure at 60°C and the resulting concentrate was lyophilized to pigment powder.
The autoxidation level of linoleic acid was measured using the method described by Cakmak et al. [26]. An end autoxidation product, MDA, was measured at 532 nm.
The reaction system of the control group (18 replicates) consisted of 1 ml 3.6 mmol/l linoleic acid, 2.0 ml distilled water, 10 μl distilled water and 10 μl 50 mmol/l FeSO4. The reaction system of AFR and ABR group (18 replicates each group) consisted of 1 ml 3.6 mmol/l linoleic acid, 2.0 ml distilled water, 10 μl anthocyanins-dimethyl sulfoxide (DMSO) solution (0.1 g/l, final concentrations) and 10 μl 50 mmol/l FeSO4. All centrifuge tubes filled with the reaction system were treated with a water bath of 37℃. Nine centrifuge tubes (3 each group) were removed, and 2 ml 0.67% (w/v) TBA was added, every 30 min. After shaking, the tubes were placed in boiling water for 30 min. and then placed in ice water to stop the reaction. Ultimately, all the tubes were centrifuged at 3 000 g for 10 min. MDA in the supernatant was measured by a spectrophotometer (Beckman, DU70) at 532 nm.
The ability of AFR and ABR to scavenge H2O2 was measured according to the method described by Ruch, et al. [27]. Fifty microliters of AFR-DMSO or ABR-DMSO solution was added to 2.95 ml H2O2 (3%, v/v) to prepare the solutions with final concentrations of 6.25, 12.5, 25, 50, 100 and 200 mg/l. The wavelength patterns (200 nm-800 nm) of AFR and ABR at different concentrations were recorded.
Superoxide anion produced by the autoxidation of epinephrine was measured according to the method of Misra, et al. [28]. The anthocyanin-DMSO solutions were added to 60 mmol/l carbonate buffered saline to prepare 6.25, 12.5, 25, 50, 100 and 200 mg/l AFR and ABR. The reaction was initiated by adding epinephrine (final concentration = 1.2 mmol/l). Then, the samples were shaken on a rotary shaker at 150 g for 5 min at 28°C. The absorbance of each sample was measured every 10 min at 480 nm by a spectrophotometer (Beckman, DU70).
2.3. Statistical analyses
All experiments were conducted in triplicate. The values are expressed as the means ± SD. The results were subjected to a one-way analysis of variance (ANOVA) with Duncan’s multiple range test for means at p<0.05. Data were processed using SigmaPlot software (version 12.0, SYSTAT Software Inc., Richmond, CA, USA).
3.1. Adsorbent capabilities of different resins and antioxidant capacity of AFR and ABR
The AFR adsorption capacities of the five resins were evaluated and are shown in Fig. 1A. The results showed that the five resins could be ranked as follows based on their adsorption capacities for AFR:AB-8>D4020>D101A>D3520>NKA-9. The adsorption ability of AB-8 was significantly stronger than that of the other resins. In addition, the polar resin NKA-9 showed the worst adsorptive ability for AFR, which was significantly different than the other resins. There were no significant differences of the adsorption capacities between D-4020 and D101A. The resins were excellently adsorbent materials for anthocyanins and generally showed different adsorbent capability to different anthocyanins [29]. Because anthocyanins are a group of phenolic compounds and have different combinations and structures in different plants, selecting the proper resin to purify AFR is necessary. Our results demonstrated that the AFR adsorption capacities of the test resins were determined by a combination of their polar, pore radius and surface area. The low polar, medium pore radius (130-140 Å) and medium surface area (480-520 m2/g) resin AB-8 showed the best adsorption capacity to AFR (Table 1 and Fig. 1A). Among the nonpolar resins, the AFR adsorption capacities mainly depended on the pore radius. These findings were consistent with the results reported by Liu et al. [30]. MDA, the autoxidation product of linoleic acid, increased quickly with treatment time in the control group (i.e., without any extracts, Fig. 1B). Both AFR and ABR distinctly restricted the autoxidation of linoleic acid. After 2.5 h, the content of MDA of the control group was 6 times higher than that of AFR and ABR. It seems that the inhibitive capacities of AFR and ABR to the autoxidation of linoleic acid were not significantly different, although the inhibitive capacity of ABR was slightly higher than that of AFR.
Table 1. Physical and chemical properties of resins.
|
Resin |
Pore radius (Å) |
Surface area (m2/g) |
Particle diameter (mm) |
Polarity |
|
D3520 |
85 ∼90 |
480 ∼ 520 |
0.3 ∼ 1.25 |
Nonpolar |
|
D4020 |
100 ∼ 105 |
540 ∼ 580 |
0.3 ∼ 1.25 |
Nonpolar |
|
NKA-9 |
155 ∼ 165 |
250 ∼ 290 |
0.3 ∼ 1.25 |
Polar |
|
AB-8 |
130 ∼ 140 |
480 ∼ 520 |
0.3 ∼ 1.25 |
Low polar |
|
D101A |
90∼ 100 |
500∼ 550 |
0.3 ∼ 1.25 |
Nonpolar |
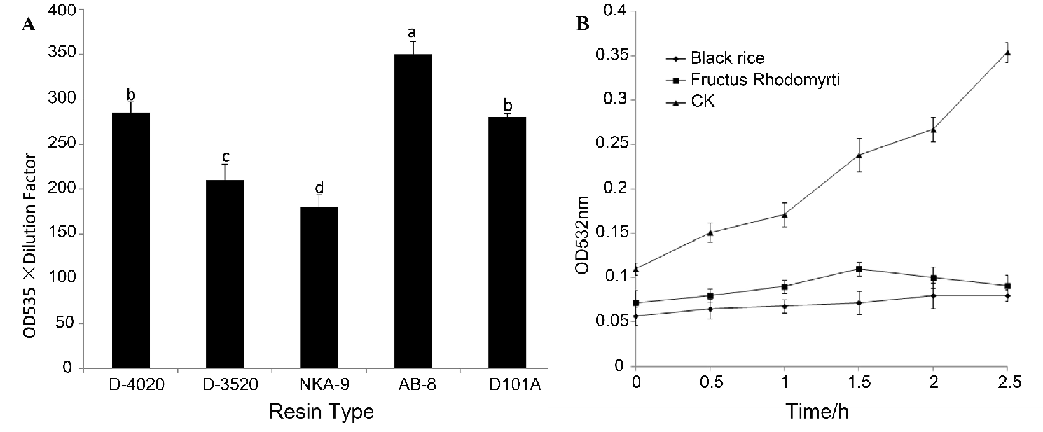
Fig. 1: (A) Adsorbent capabilities of different resins for anthocyanins from fructus rhodomyrti. (B) Inhibitive capacity of the autoxidation of linoleic acid by AFR or ABR. CK was the control group without any extracts (means ± SD, n = 6).
The wavelength patterns (200 nm-800 nm) of different concentrations of AFR and ABR (6.25, 12.5, 25, 50, 100 and 200 mg/l) are shown in Fig. 2. The different spaces between each curve at 230 nm represented the different scavenging activities for H2O2 by each extract. The spaces between the curves of AFR were larger than those of ABR, which indicated that the scavenging activity of ABR to H2O2 was stronger than that of AFR.
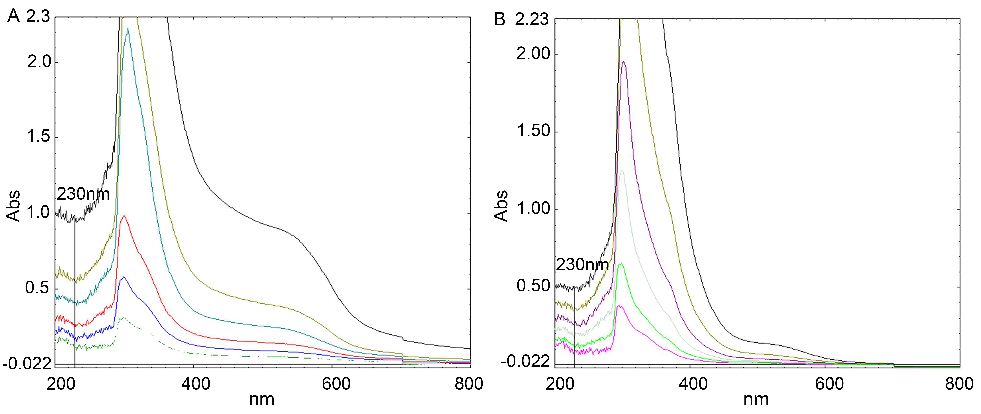
Fig. 2: Wavelength patterns (200 nm-800 nm) with different concentrations of AFR and ABR (from bottom to top: 200, 100, 50, 25, 12.5, and 6.25 mg/l)
Adrenochrome, the autoxidation production of epinephrine treated with AFR and ABR, was measured by a spectrophotometer at 480 nm. The results are shown in Fig. 3. The values of adrenochrome absorbance (OD480nm) linearly decreased when epinephrine was treated with AFR or ABR. The linear regression results showed that the absolute value of the slope of the ABR group (0.0336) was higher than that of AFR (0.0172), which indicated that the inhibitive capacity of the autoxidation of epinephrine by ABR was stronger than that of AFR.
The purified AFR anthocyanins showed strong antioxidative activities in this study. It has been reported that the oxygen radical absorption capacity of AFR is higher than that of commonly consumed fruits, such as mangoes, kiwis, red grapes, tangerines, cherries, apples, and bananas [31]. Therefore, fructus rhodomyrti is usually considered an excellent underexploited nutraceutical. Cyanidin-3-O-glucoside has been considered the most abundant anthocyanin both in AFR and ABR [32]. Based on our results, the scavenging activity of AFR anthocyanins for H2O2 and the inhibitive capacity to the autoxidation of linoleic acid and epinephrine were all slightly weaker than those of ABR. Therefore, from an antioxidant perspective, AFR is not better than ABR, which is probably one of the reasons that fructus rhodomyrti has not attracted much attention to date.
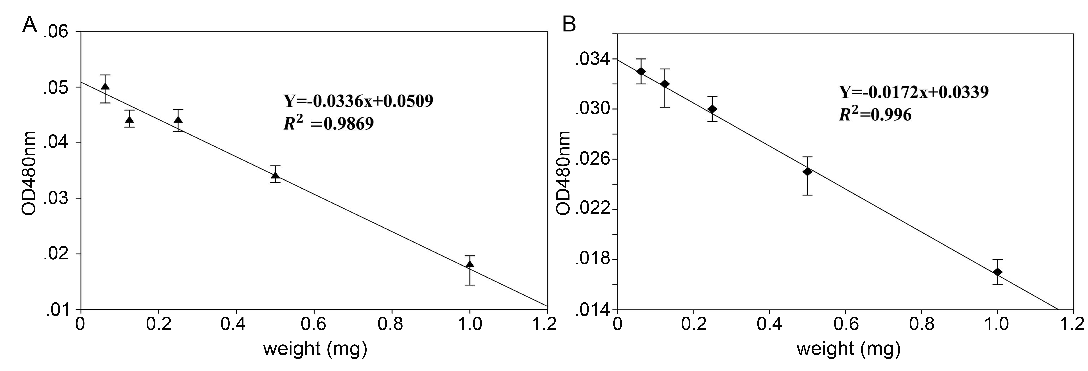
Fig. 3: OD value of epinephrine treated with different concentrations of AFR and ABR (means ± SD, n = 6).
In addition, fructus rhodomyrti is the fruit of Rhodomyrtus tomentosa, which is a native dominant shrub in subtropical shrublands. As a nurse plant, Rhodomyrtus tomentosa facilitates the survival of many native species and their population of degraded lands, and it also plays an important role in early forest successional stages [33]. However, both the natural fruit yield and seed germination rate of wild Rhodomyrtus tomentosa are relatively low. Therefore, based on an ecological perspective, it is still ambiguous whether fructus rhodomyrti would be suitable for use as a popular nutraceutical.
From the nutrient perspective, fructus rhodomyrti is an excellent, but underexploited, nutraceutical material. However, considering the restorative function of Rhodomyrtus tomentosa in early successional stages and the near equivalence of its antioxidant activities with those of black rice, we still suggest the use of black rice instead of a fructus rhodomyrti product for the dietary intake of anthocyanins in everyday health care.
This work was supported by the National Natural Science Foundation of China (31870374 and 31570398).
Münzel T., Camici G.G., Maack C., Bonetti N.R., Fuster V. and Kovacic, J.C., 2017. Impact of oxidative stress on the heart and vasculature. J. Am. Coll. Cardiol., 70: 212-229 PMid:28683969
View Article PubMed/NCBIPrasad S., Gupta S.C. and Tyagi A.K., 2017. Reactive oxygen species (ROS) and cancer: role of antioxidative nutraceuticals. Cancer Lett., 387: 95-105 PMid:27037062
View Article PubMed/NCBINg T., Liu F. and Wang Z.T., 2000. Antioxidative activity of natural products from plants. Life Sci., 66: 709-723 00642-6
View ArticlePérez-Gregorio M.R., Regueiro J., Simal-Gándara J., Rodrigues A.S. and Almeida D.P., 2014. Increasing the added value of onions as a source of antioxidant flavonoids: a critical review. Crit. Rev. Food Sci. Nutr., 54: 1050-1062 PMid:24499121
View Article PubMed/NCBIAriza M.T., Reboredo-Rodríguez P., Cervantes L., Soria C., Martínez-Ferri E., González-Barreiro C., Cancho-Grande B. and Battino M., Simal-Gándara J., 2018. Bioaccessibility and potential bioavailability of phenolic compounds from achenes as a new target for strawberry breeding programs. Food Chem., 248: 155-165 PMid:29329839
View Article PubMed/NCBIKulakowski D., Leme-Kraus A.A., Nam J.W., McAlpine J., Chen S.N., Pauli GF., Ravindran S. and Bedran-Russo A.K., 2017. Oligomeric proanthocyanidins released from dentin induce regenerative dental pulp cell response. Acta Biomater., 55: 262-270 PMid:28365481
View Article PubMed/NCBIOmori G., Yamamoto N., Fushimi T., Kawasaki K. and Oota N., 2017. Effects of combined intervention of quadriceps exercise and green tea catechins on physical function in medial knee osteoarthritis. Osteoarthr. Cartilage, 25: S398
View ArticleFigueiredo-González M., Reboredo-Rodríguez P., González-Barreiro C., Simal-Gándara J., Valentão P., Carrasco-Pancorbo A., Andrade P.B. and Cancho-Grande B., 2018. Evaluation of the neuroprotective and antidiabetic potential of phenol-rich extracts from virgin olive oils by in vitro assays. Food Res. Int., 106: 558-567 PMid:29579961
View Article PubMed/NCBIFigueiredo-González M., Reboredo-Rodríguez P., González-Barreiro C., Carrasco-Pancorbo A., Simal-Gándara J. and Cancho-Grande B., 2018. Nutraceutical potential of phenolics from 'Brava' and 'Mansa' extra-virgin olive oils on the inhibition of enzymes associated to neurodegenerative disorders in comparison with those of 'Picual' and 'Cornicabra'. Molecules, 23: 1-14 PMid:29561824
View Article PubMed/NCBIReboredo-Rodríguez P., González-Barreiro C., Cancho-Grande B., Simal-Gándara J., Giampieri F., Forbes-Hernández T.Y., Gasparrini M., Afrin S., Cianciosi D., Manna P.P., Varela-López A., Ojeda-Amadorc R.M., Fregapanec G., Salvadorc M.D. and Battino M., 2018. Effect of pistachio kernel extracts in MCF-7 breast cancer cells: inhibition of cell proliferation, induction of ROS production, modulation of glycolysis and of mitochondrial respiration. J. Funct. Foods, 45: 155-164
View ArticlePerez-Gregorio M.R. and Simal-Gandara J., 2017. A critical review of the characterization of polyphenol-protein interactions and of their potential use for improving food quality. Curr. Pharm. Des., 23: 2742-2753 PMid:28155599
View Article PubMed/NCBIReboredo-Rodríguez P., González-Barreiro C., Cancho-Grande B., Forbes-Hernández T.Y., Gasparrini M., Afrin S., Cianciosi D., Carrasco-Pancorbo A., Simal-Gándara J., Giampieri F. and Battino M., 2018. Characterization of phenolic extracts from Brava extra virgin olive oils and their cytotoxic effects on MCF-7 breast cancer cells. Food Chem. Toxicol., 119: 73-85 PMid:29753866
View Article PubMed/NCBIKong J.M., Chia L.S., Goh N.K., Chia T.F. and Brouillard R., 2003. Analysis and biological activities of anthocyanins. Phytochemistry, 64: 923-933 00438-2
View ArticleCerezo A.B., Cuevas E., Winterhalter P., Garcia-Parrilla M.C. and Troncoso A.M., 2010. Isolation, identification, and antioxidant activity of anthocyanin compounds in Camarosa strawberry. Food Chem., 123: 574-582
View ArticleDachriyanus, S. Sargent M.V., Skelton B.W., Soediro I., Sutisna M., White A.H. and Yulinah E., 2002. Rhodomyrtone, an Antibiotic from Rhodomyrtus tomentosa. Aust. J. Chem., 33: 229-232
View ArticleGeetha K.M., Sridhar C. and Murugan V., 2010. Antioxidant and healing effect of aqueous alcoholic extract of Rhodomyrtus tomentosa (Ait.) Hassk on chronic gastric ulcers in rats. J. Pharm. Res., 3: 2860-2862.
Chen T., Yu C. and Yang B., 2011. Structure elucidation and NMR assignments for two new quinones from Fructus rhodomyrti of Rhodomyrtus tomentosa. Chem. Nat. Comp., 47: 524-526
View ArticleLai T.N., André C., Rogez H., Mignolet E., Nguyen T.B. and Larondelle Y., 2015. Nutritional composition and antioxidant properties of the sim fruit (Rhodomyrtus tomentosa). Food Chem., 168: 410-416 PMid:25172728
View Article PubMed/NCBIAfrin S., Giampieri F., Cianciosi D., Pistollato F., Ansary J., Pacettia M., Amicia A., Reboredo-Rodríguez P., Simal-Gandara J., Quiles J.L., Forbes-Hernández T.Y. and Battino M., 2019. Strawberry tree honey as a new potential functional food. Part 1: Strawberry tree honey reduces colon cancer cell proliferation and colony formation ability, inhibits cell cycle and promotes apoptosis by regulating EGFR and MAPKs signaling pathways. J. Funct. Foods, 57: 439-452
View ArticleAfrin S., Forbes-Hernández T.Y., Cianciosi D., Pistollato F., Zhang J.J., Pacettia M., Amicia A., Reboredo-Rodríguez P., Simal-Gandara J., Bompadre S., Quiles J.L., Giampieri F. and Battino M., 2019. Strawberry tree honey as a new potential functional food. Part 2: Strawberry tree honey increases ROS generation by suppressing Nrf2-ARE and NF-кB signaling pathways and decreases metabolic phenotypes and metastatic activity in colon cancer cells. J. Funct. Foods, 57: 477-487
View ArticleCai S.J. Huang R., Zhang S. and Jiang X., 2008. Study on antioxidation capacity of extracts from fructus rhodomyrti. Modern Food Sci. Technol., 24: 1229-1231.
Ke Y., Xu X., Wu S., Huang J., Geng Y., Misra H. and Li Y., 2013. Protective effects of extracts from Fructus rhodomyrti against oxidative DNA damage in vitro and in vivo. Oxid. Med. Cell. Longev., 2013: 507407 PMid:24089629
View Article PubMed/NCBIFernandes F., Pereira E., Prieto M.A., Calhelha R.C., Ćirić A., Soković M., Simal-Gandara J., Barros L. and Ferreira I.C.F.R., 2019. Optimization of the extraction process to obtain a colorant ingredient from leaves of Ocimum basilicum var. purpurascens. Molecules, 24: 686-704 PMid:30769867
View Article PubMed/NCBIda Silva L.P., Pereira E., Prieto M.A., Simal-Gandara J., Pires T.C., Alves M.J., Calhelha R., Barros L. and Ferreira I.C., 2019. Rubus ulmifolius Schott as a novel source of food colorant: extraction optimization of coloring pigments and incorporation in a bakery product. Molecules, 24: 2181 PMid:31185684
View Article PubMed/NCBILohachoompol, V. Srzednicki G. and Craske J., 2004. The change of total anthocyanins in blueberries and their antioxidant effect after drying and freezing. J. Biomed. Biotechnol., 5: 248-252 PMid:15577185
View Article PubMed/NCBICakmak I. and Horst W.J., 1991. Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol. Plantarum, 83: 463-468
View ArticleRuch R.J., Cheng S.J. and Klaunig J.E., 1989. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis, 10: 1003-1008 PMid:2470525
View Article PubMed/NCBIMisra H.P. and Fridovich I., 1971. The generation of superoxide radical during the autoxidation of ferredoxins. J. Biol. Chem., 246: 6886-6890
Schwarz M., Hillebrand S., Habben S., Degenhardt A. and Winterhalter P., 2003. Application of high-speed countercurrent chromatography to the large-scale isolation of anthocyanins. Biochem. Eng. J., 14: 179-189 00219-X
View ArticleLiu X., Xiao G., Chen W., Xu Y. and Wu J., 2004. Quantification and purification of mulberry anthocyanins with macroporous resins. Biochem. Eng. J., 5: 326-331 PMid:15577197
View Article PubMed/NCBIWu X., Beecher G.R., Holden J.M., Haytowitz D.B., Gebhardt S.E. and Prior R.L., 2004. Lipophilic and hydrophilic antioxidant capacities of common foods in the United States. J. Agr. Chem., 52: 4026-4037 PMid:15186133
View Article PubMed/NCBICui C., Zhang S., You L., Ren J., Luo W., Chen W. and Zhao M., 2013. Antioxidant capacity of anthocyanins from Rhodomyrtus tomentosa (Ait.) and identification of the major anthocyanins. Food Chem., 139: 1-8 PMid:23561070
View Article PubMed/NCBILiu N., Hai Ren H., Yuan S., Guo Q. and Wang L., 2013. Testing the stress-gradient hypothesis during the restoration of tropical degraded land using the shrub Rhodomyrtus tomentosa as a nurse plant. Restor. Ecol., 21: 578-584
View Article