Felix Akharume
Email: Felix.Akharume@uky.edu
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 4 ISSUE: 9
Page No: 956-969
Felix Akharume
Email: Felix.Akharume@uky.edu
Felix Akharume1, Alexandra Smith3, Litha Sivanandan2, and Kaushlendra Singh1
1School of Natural Resources, West Virginia University
2 Extension Service (Families and Health Programs), West Virginia University, Morgantown, USA
3PepsiCo, Baltimore, Maryland Area, USA
Liyou Zheng(liyou890513@sina.com)
R%c3%b3n%c3%a1n Doherty(ronan.doherty@lyit.ie)
Biljana Pokimica(biljana.pokimica@hotmail.com)
Felix Akharume, Alexandra Smith, Litha Sivanandan, and Kaushlendra Singh, Recent Progress on Osmo-convective Dehydration of Fruits(2019) Journal of Food Science & Technology 4(9) p:956-969
There are continuous research and industrial application for osmo-convective dehydration as a choice technology for the processing of dried fruit snacks and the development of new functional fruit snacks. Owning to the quality loss and a high energy investment associated with the convective dehydration of fresh fruits into dry snacks; osmotic dehydration prior to convective dehydration has become widely used for quality improvement, energy conservation and production of functional dried fruits snacks. In this review, we address the current knowledge in the use of osmo-convective dehydration to produce dried fruit snacks by providing information on the optimized process condition for producing such dried fruit snacks. Additionally, we present a summary of information on the effects of osmo-convective dehydration on the shelf life, nutritional quality, and microbial stability of dried fruit snacks. Finally, we provide information on the potential application of osmo-convective dehydration in developing functional fruits.
Keywords: osmo-convective dehydration, fruits, quality, nutrition, product development
The world fruit production was estimated to be about 881.2 million metric tons in 2017 (FAO, 2019) while in the US about 23.55 million metric tons of fruit were produced in 2016 (USDANASS, 2017) and 23.88 million metric tons of fruit were produced in 2017 (FAO, 2019). Fruits consumption is highly recommended for the general well-being and health of consumers. The U.S. Departments of Health and Human Services (HHS) and of Agriculture (USDA) have recommended increased intake of fruits and vegetables in addition to grains, fat-free or low-fat dairy, a variety of protein, and oil all within an appropriate calorie level (USDA & USDHHS, 2019). However, about seventy-five percent of Americans still comsumes fruits below the recommendations of the Dietary Guidelines for Americans (DGA). The majority of Americans still get their energy from fat and added sugar level (McNamara et al., 1999; USDA & USDHHS, 2019). In view of this trend, there is a need for an increased supply of fruits in order to meet the projected dietary recommendation for the year 2020 and beyond. It has been projected that by the year 2020, the largest food supply gap is expected in fruits (McNamara et al., 1999). About ninety-four percent increase in fruit supply from the fruit supply in the year 1994 (77,512 million pounds) will be needed to met the recommended recurement by the year 2020 (McNamara et al., 1999).
Seasonality in fresh fruit production and post-harvest losses due to perishability have been identified as the main factors contributing to the unavailability and inaccessibility to fruits. In fact, Blanchette and Brug (2005) agreed that this factor was the most consistent determinant for the lack of adequate fruit consumption among 6 - to 12-year-old children (Blanchette & Brug, 2005). Thus, fruit preservation is a widely used approach to ensure availability by increasing the shelf life of fruits. Preservation minimizes fruit deterioration, eradicate fruit waste, ensure fruit availability all year round, and provide safe and nutritive fruits to the consumers. Additionally, processing fresh fruits into new value-added products or as part of other food matrices are other ways to extend the shelf life of fruits and increase their availability. Drying, particularly through hot air is one of the oldest and widely used methods of preservation that have been widely used to ensure a continuous supply of fruits in dried form. However, the hot-air (convective) drying process is associated with some inherent challenges such as long drying times, high cost of energy input, and low quality of the dried products (Zhang et al., 2017). Thus, the hot-air drying process is often combined with osmotic dehydration as a pretreatment to improved the dried product quality, save cost and reduce the overall energy investment.
Osmotic dehydration prior to drying is a widely adopted pretreatment method because of its advantages over the other pretreatments as will be discussed later in this review. The combined process of osmotic dehydration followed by a convective drying step for food processing and preservation is termed “Osmo-convective dehydration ” or sometimes referred to as osmotic-air dehydration in some literature (Tiwari, 2005; Yu, 1998). Osmo-convective dehydration technology combines the advantages of the two dehydration techniques: “osmotic dehydration” and “convective drying” to develop dried fruits snacks with enhanced quality, taste, and consumer appeal. Therefore, in this review article, we present the current knowledge on the use of osmo-convective dehydration technology in the development of dried fruit snacks. First, we looked at the two-unit processes that make up this technology separately and then together. Finally, we addressed the use of osmo-convective dehydration as preservation techniques and the impacts of this technology on the nutrient and microbial quality of the dried fruit snacks.
2. Current Status of Knowledge on Osmotic Dehydration
2.1 Osmotic dehydration
Osmotic dehydration (OD) is a well-known fruit dehydration and preservation technique. It works on the well-established principle of lowering water activity by the addition of humectants and removal of water from the food (Lewicki & Lenart, 1995). Osmotic dehydration has been well defined by several authors as a phenomenon that exists, wherein a solid food material is immersed in a hypertonic solution, usually a solution of edible solute (such as sugar, salt, fructose, high fructose corn syrup (HFCS), maltodextrin, sorbitol, additives, or combination of these). As a result of differential osmotic concentration, a counter-current flow of water from the food into the solution and solutes from the solution into the solid food through a non-selective semi-permeable membrane occurs. This phenomenon is also trailed by leaching of a negligible amount of solute (sugar, organic acid, vitamins, minerals, salts, fragrances, colorants, etc.) from the food into the solution (Rastogi et al., 2002; Torreggiani, 1993). The flux of water removal is considerably larger than the flux of solutes infusion, this explains the reason why the term “dehydration” is added to the term “osmosis” to form “osmotic dehydration. Osmotic dehydration has so many advantages over-drying or other methods of moisture removal from fruit because of the following reasons:
Notwithstanding these advantages, OD is considered a partial water removal technique and so, there is always a need for an additional dehydration technique if the shelf life of the fruits is to be extended. For instance, minimally processed fruits obtained through OD have about 20-30% moisture reduction (Khan, 2012; Yadav & Singh, 2014). Similarly, intermediate moisture fruits obtained from OD have about 30 - 70% moisture reduction (Khan, 2012; Yadav & Singh, 2014). These OD products are not shelf-stable for a long time (maximum for 21 days) due to high water activity (0.92 -0.97) (Ahvenainen, 1996; Moreno et al., 2000).
2.2 Mechanism of osmotic dehydration
Osmotic dehydration is based on the principle of osmosis (Figure 1), wherein there is mass transfer through a semi-permeable membrane powered by the concentration gradient across the membrane. In osmotic dehydration of fruits, the fruit cell walls act as the semipermeable membrane allowing the exchange of water and low molecular solutes (Shi & Le Maguer, 2002). Thus, due to the low permeability of this membrane, solutes impregnation occurs closer to the surface of the fruit and not at the core of the fruit. However, the water molecules are being removed across the fruit matrix by capillary flow and diffusion (Ahmed et al., 2016; Chiralt & Talens, 2005). On one side of the fruit cell membrane is the vacuole, which contains a cell sap of a certain concentration. The cell sap has its osmotic pressure exerted on the protoplasm and the cell wall making the cell turgid (Lewicki & Lenart, 1995). On the other side of the membrane is the osmotic solution with a higher concentration and pressure against the cell wall. Owning to this pressure difference, there occurs an exchange of water from the fruit and solutes from the surrounding osmotic solution. Fruits, like other biological material, are made of heterogeneous tissues with connecting cells and micro-pores and as such, the mass transfer during OD processes occurring throughout the fruit matrix and across the semi-permeable membrane is facilitated by both diffusion and capillary flow (Khan, 2012; Shi et al., 2009). Earlier works on OD often consider the fruit as a macroscopic matrix of homogenous tissues (Le Maguer & Yao, 1995; Maguer, 1997; Salvatori et al., 1998). It was assumed that diffusion is the only mechanism of mass transport during OD processing and that a superficial transport front exists only within 2-3 mm depth of the fruit matrix, causing a barrier to subsequent solute impregnation from the OD solution and exodus of soluble solute and water from the fruit (Marcotte & Le Maguer, 1992; Raoult-Wack et al., 1991; Rastogi et al., 2002). As such, the OD process was explained and modeled using Fick’s second law of unsteady-state diffusion without recourse to capillarity (Azuara et al., 1992; Fick, 1855). However, recent works on OD explored the microscopic approach to understanding the mass transfer mechanism. Salvatori et al., (1998) propose the concept of “advance disturbance front’ (ADF) to explain the mass exchange mechanism during OD, especially at room temperature. The ADF is described as a virtual plane separating layers of fruit with non-significant mass transfer (Salvatori et al., 1998). They submitted that, with a long OD time, it is possible to extend ADF to a depth of 15mm from the fruit-solution interface. Rastogi research group reported a similar possibility, explaining that the state of the cell membrane does not remain constant as earlier assumed but it changes with osmotic stress. The cell membrane could change from semi-permeability to full permeability state allowing the dehydrating front to proceed to the center of the fruit from the surface (Rastogi et al., 2002). At the beginning of OD, the tissue in contact with the OD solution exudes water through diffusion, ruptures, and shrinks. Consequently, this dead cell increases the permeability index of the fruit cell wall and increases its diffuson rate. As OD progresses, the dehydrating front moves inward to the core of the fruit, causing a disintegration of the already dehydrated tissues. The rate of diffcuion here is much lower than the rest. This makes it possible for water to diffuse through the whole matrix of the fruit through both diffusion and capillary flow (Rastogi et al., 2000; Rastogi et al., 2002).
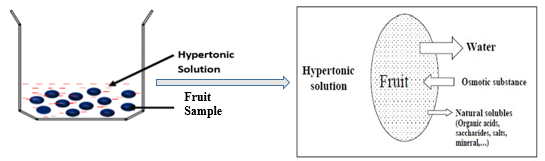
Figure 1. Schematic diagram of the osmotic dehydration process
Unlike previous study that assumes contant rate of diffusion during OD, Rastogi et al (2002) identifies three water diffuson rates (D1, D2, and D3); D1 accounts for diffusion from the core of the fruit to the dehydrating front, D2 reprents diffusion coefficient across the dehydrating front to the fruits surface layer and D3 is the diffusion coefficient from the fruit surface to the sosmotic solution.
In some cases, when the OD process is carried out under vacuum, the proposed mass transfer model is Hydrodynamic Mechanism (HDM) (Corrêa et al., 2010; Fito, 1994; Fito & Pastor, 1994). The HDM results from a combination of pressure gradients and capillary flow imposed on the fruit-solution system (Corrêa et al., 2010; Fito, 1994; Fito & Pastor, 1994). Fruit as porous material is made up of gas occluded in the intercellular spaces. When a vacuum is applied, there is a lower pressure in the fruit-solution system compared to the atmospheric pressure. Thus, the occluded gas expands and escapes from the fruit creating room for the capillary inflow of surrounding solution when pressure is restored, which dehydrates and impregnates the fruits simultaneously (Sagar & Kumar, 2010).
2.3 Effect of process conditions during osmotic dehydration
Osmotic dehydration, either as a drying pretreatment or as a separate dehydration technique, has the unique advantage over all other dehydration and pretreatment methods. OD provides an opportunity to control, modify or create a new formulation depending on the desired quality intended for the final products. Several process parameters can be controlled to achieve the desired product during OD process such as type of solution/osmo-active agents, composition and concentration of solution, molecular weight of osmo-active agents, fruit to solution ratio, fruit geometry, fruit size and maturity, OD temperature, OD time, ambient pressure (atmosphere or vacuum), degree of agitation, OD equipment as well as the type of pretreatment if OD is used as a separate dehydration technique. From the onset of the application of OD to food, there has been a continuous stream of publication and patents exploring the permutation of these parameters for product and process optimization.
2.3.1 Osmotic solution type and concentration
The nature of the osmotic solution is an important factor in the development of an OD product. The type of solution/osmo-active agents, the concentration of the solution and the molecular weight of osmo-active agents strongly influence the osmotic gradient which is the driving force for the OD process. Consequently, the water loss and solid gain (WL/SG) index, the water activity, and the overall quality of the OD product are affected. Shi et al., (2009) studied the effect of different osmotic agents (fructose, dextrose, polydextrose, sucrose, maltodextrin, and corn syrup) on the solid gain in blueberries under dynamic infusion method. Their result shows that monosaccharide (fructose, dextrose) caused a higher solid gain (about 1.3 and 1.4 g/ g of dry solid) than polysaccharides (polydextrose was about 1.2 g/g, corn syrup about 0.9 g/g, and maltodextrin about 0.7 g/g) for the same concentration and process condition (240 min). The explanation for this was that monosaccharides with lower molecular weight have a higher diffusion coefficient than disaccharides (Shi et al., 2009).
The fact that the concentration and molecular weight of the solute in the OD solution correlate positively with increased mass transfer has been corroborated by several works (Azoubel & Elizabeth Xidieh Murr, 2004; Khan et al., 2011; Lazarides et al., 1995; Nsonzi & Ramaswamy, 1998; Sacchetti et al., 2001). The concentration of the solution is known to affect the WL/SG index up to a certain level beyond which there will not be a significant difference in the WL/SG index. Giraldo et al., (2003) reported an increase in water transfer rate with OD solution concentration when mango fruit was treated in a sucrose solution with a concentration range of 35-65 °Brix at 30 ℃ under atmospheric pressure. However, the increase was only observed from 35 - 45 °Brix, but not 55 and 65°Brix (Giraldo et al., 2003). Therefore, depending on the type of fruits and process condition, the WL and SG may only increase significantly with concentration up to a certain point. For example, there was non-significant water loss or solid gain in pumpkin osmosed with 55% and 65% sucrose solution after 4 h of OD (Pan et al., 2003). As such a careful combination of OD parameters (concentration, time, temperature, etc.) is needed for optimization. While an increase in solution concentration correlates positively with an increase in solid gain and water loss up to a certain point, it however negatively correlates with average diffusion coefficients for water loss and solid gained. In a separate experiment, where apple slices where osmosed with 40, 50, 60 % sucrose solution at 27℃, effective diffusion coefficients for water loss were 35.930, 13.282, 7.603 x 10 11 m2/s, respectively and solid gain was 2.174, 0.939, 0.264 x 10 11 m2/s, respectively (Rodrigues & Mauro, 2008). Another factor worthy of note is the osmotic solution pH. The pH concentration of the solution influences the mass transfer rate during OD by modifying the fruit cell architecture. Allali et al., (2010) performed osmotic dehydration of apple cubes using a solution of a 15% w/w aqueous sucrose solution in addition to 0 - 2% citric acid. Their result showed a consistent increase in water loss and solid gain in OD process containing varying concentration of citric acid compared to others treatments without citric acid. For instance, water loss was 48% after 1 h of OD in treatemsts containing 2% citric acid and 50% after 4 h of OD in treatment without citic aicd. In a similar but different experiment, Mauro et al., (2016) performed an OD experiment fo cylinder shaped apples using 40% aqueous sucrose solution in addition to 2% ascorbic acid. The result showed that water loss and solid gain increased when compared to OD with sucrose only at all OD times. For instance, a water loss of 10.45g/100g and a solid gain of 3.18g /100g of apple fruit was recorded in OD solution containing 40% aqueous sucrose solution in addition to 2% ascorbic acid after 30 min while a water loss of 9.36 g/100g and a solid gain of 2.28g /100g of apple fruit was recorded in OD solution containing only 40% aqueous sucrose solution. Both research groups affirmed that the increase in water loss and solid gain recorded was due to the presence of these acid ingredients which may have modified the fruit cellular structure (Allali et al., 2010; Mauro et al., 2016). Similarly, the addition of salt to the OD solution has been known to facilitate the mass transfer. Similarly, the addition of salt to the OD solution has been known to facilitate the mass transfer. Singh et al., (2017) reported an increase in water loss and solid gain when the salt concentration in a 50 °Brix sucrose solution increased from 5% to 15% (Singh et al., 2007).
2.3.2 Fruit characteristic, fruit to solution ratio, and degree of agitation
Other parameters such as fruit to solution ratio, fruit geometry, fruit size, and maturity as well the degree of agitation of the whole system affect the ratio of SG/WL, diffusion coefficient, and quality of the products. It is evident from the different model describing OD process, that the thickness of the fruits varies inversely to the concentration gradient in fruit (SG at initial/SG at infinity or WL at initial /WL at infinity)(Azuara et al., 1992; Crank, 1975; Ochoa-Martinez et al., 2007; Peleg, 1988). This evidence is supported by the works of Panagiotou et al (1998) in which osmotic dehydration of apples, banana, and kiwi fruits was carried out at a temperature of 40℃, sucrose concentration of 40% and under atmospheric pressure. The thickness of apple slabs was maintained at 4, 8, 12, 16 and 20 mm. The kiwi fruit samples were formed from two half-slab samples, and the average diameter for kiwi fruit and banana was 4.5 cm and 2.2 cm respectively. Their result shows that the thickness of the fruits negatively correlated to water WL and SG (Panagiotou et al., 1998). In the same experiment, they further reported that the rate of agitation correlates positively to the rate of WL and SG. Additionally, they reported that with a faster speed of agitation, the equilibrium time (6 h) of small sizes apples, banana, and kiwi fruit of less than 4 mm can be reduced. Mathematical models of different fruits geometry used in OD has been extensively researched and compiled (Assis et al., 2016; Crank, 1975; Ochoa-Martinez et al., 2007). Theoretically, it is known that the more surface area of the fruit in contact with the solution, the higher the rate of mass transfer facilitated (Panagiotou et al., 1998; van Nieuwenhuijzen et al., 2001).
Depending on the objectives of osmotic dehydration (dewatering or impregnation), the fruit to solution ratio influences the rate of WL and SG. Decreasing the “fruit to solution ratio” increases WL and SG (Singh et al., 2007). However, there was a deviation from this theory with the experiment on the OD of carrot (Singh et al., 2008). It was observed that when carrot cubes were osmosed in sodium chloride solution having concentrations of 5%, 10%, and 15% (w/v), at solution temperatures of 35, 45, and 55℃, sample to solution ratios 1:4, 1:5 and 1:6, for 240 min., there was no significant difference (P>0.05) in the amount of solid gained or water loss due to “fruit-solution ratio”. The increases in SG or WL were attributed to the temperature effect (Singh et al., 2008). Fruit to solution ratio range of 1:4 to 1:6 is recommended for a balance of water loss and solid gain (Khan et al., 2011). The nature of the fruits (fresh or frozen, degree of ripeness, time of storage prior to OD) is another factor to be considered. In an experiment conducted by Akharume et al., (2016) on osmotic dehydration of fresh and frozen blueberries osmosed in a 42 °Brix solution at 50℃ for 0- 10 h, the group recorded three times as much solid gain and four times as much water loss in the frozen blueberries compared with the fresh ones (Akharume et al., 2016).
2.3.3 Temperature, time, and pressure
Effects of temperature, OD process time, ambient pressure (atmosphere or vacuum) are the major process parameters in OD that influence the kinetics of WL and SG. Nsonzi and Ramaswamy (1998), measured the effect of these parameters (time, temperature) under atmospheric pressure in osmotic dehydration of blueberries at varying sucrose concentration ranges (47°Brix - 70°Brix). They reported that time and temperature effects were significant on water loss (P<0.001) and on the solid gain at (P<0.05) (Nsonzi & Ramaswamy, 1998). Lazarides et al., (1995) reported similar effects for temperature - an increase in temperature resulted in an increase in WL and SG (Lazarides et al., 1995). Wang et al (2015) assayed the effect of vacuum (20 kpa) and normal pressure on WL and SG of apples. The vacuum were a single short period vacuum impregnation (30min at 20 kPa followed by a 90 min impregnation at atmospheric pressure) and an intermittent vacuum impregnation (4 cycles of 10 min at 20 kPa and 4 cycles of 20 min atmospheric pressure). Irrespective of the other process conditions (concentration and temperature), vacuum pressure favored a lower WL in the fruit compared to normal pressure and supported a higher SG compared to normal pressure. For instance, at 500Brix and temperature of 40℃, water loss of 2.21 and 1.24 g/g and solid gain of 0.39 and 0.88 g/g was recorded for both non-vacum treatment and vacuum treated OD respectively (Wang et al., 2015). In another OD experiment where apple slices were impregnated with β-carotene, pulsed vacuum osmotic dehydration condition resulted in a higher SG of 4.7, 5.5, and 6 mg β-carotene per grams of dry solid, while OD under atmospheric pressure condition yielded 1.5, 3.5, and 4.1mg β-carotene per grams of dry solid for concentration level 30, 40, and 50°Brix (Santacruz-Vázquez et al., 2008).
3. Current Status of Knowledge about Convective Drying of Fruits
3.1 Convective drying
Drying is a thermal processing technique in which heat is bought in contact with the surface of the material being dried to remove moisture(Geankoplis, 1993). Different kinds of equipment provide this technique of drying, for example, tray dryer, cabinet dryer (Ramaswamy & Nsonzi, 1998), tunnel dryer, fluidized bed dryer (Yemmireddy et al., 2013), and spray dryer (Sosa et al., 2012). In this equipment, there are common features that are peculiar and pertinent to their functionality: a heat source, a fan or blower to circulate and carry heat and housing to hold the drying materials (Figure 2). Convective drying has been widely used to dehydrate high and intermediate moisture fruit into dried fruit snacks with a moisture content of 5% (Geankoplis, 1993; Yemmireddy et al., 2013) and water activity usually lower than 0.61, where no bacterial growth is observed. However, the products from this method are often of low quality and the long drying time and high temperature cause a loss of color, flavor, and nutrient of the final dried products. Drying at temperature greater than 80°C induces Maillard and Caramelization reaction in a product at lower moisture content.
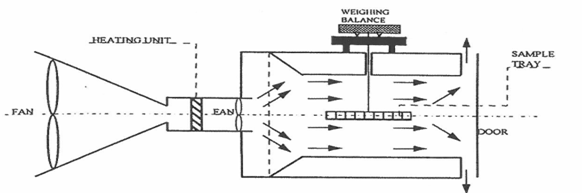
Figure 2. Schematic diagram of convective drying unit (Ramaswamy & Nsonzi, 1998)
3.2 Mechanism of convective drying
Convective drying is a common energy-intensive method of dehydration in which free water molecules are removed in the form of vapor by hot air in contact with the food material from the surface and the interior of solid material (Mehrafarin and Faghihi, 2001). The phenomena underlying this process is a complex problem involving simultaneous mass and energy transport in a hygroscopic, shrinking system (Ratti, 2001). Generally, in drying a single- or multi-component liquid gradually evaporates and is removed from the complex porous structure via combined heat and mass transfer processes. Convective drying occurs through evaporation of moisture from the exposed surface of a solid. At separate and independent times, moisture removal involves the combination of convective and diffusive flows from the depths of the solid to the surface when there is a concentration difference between the depth of the solid and the surface (Sabarez, 2016). Fruit as a biological material contains a network of cells with interconnecting pores and channels. Free moisture moves through these capillaries and pores from the depth of the porous fruit solid to the surface through capillarity action and not by diffusion (Geankoplis 1993). As the water is removed, a point is reached where there is insufficient water left to maintain continuous water film across the pores. At this point, moisture movement is through diffusion, especially in fine pore fruit solids (Geankoplis 1993).
3.3 Effect of convective drying process conditions
Drying temperature, drying time, air velocity, ambient humidity, initial moisture of the fruit, size of the fruit and types of pretreatment are the major parameters that influence mass transfer during convective drying of fruit material. Like the process condition for OD, an increase in the convective parameters (temperature, time, air velocity) positively correlates with moisture loss and weight reduction. However, the ambient humidity, the size and initial moisture content of the fruits correlates negatively with moisture loss, weight reduction and drying time.
3.3.1 Degree of air pressure, temperature, and velocity
In an experiment, to study the kinetics of convective drying of blueberry variety (O’neil variety) at three different temperatures (60, 70, and 80℃) with an airflow of 2.0 ± 0.2 m/s, the result obtained showed that drying times depend largely on the air temperature as evidence from the drying characteristic curves. Blueberries dried at 80℃ showed the lowest drying time, followed by blueberries dried at 70℃ and the next 60℃. (Vega-Gálvez et al., 2009). Similarly, in another research drying time of rabbiteye blueberries was recorded to be 50 % longer when dried at 85℃ (5.20h) compared to 107℃ in the trio of air-impingement, fluidized bed, and forced air dryer to reach aw of 0.55 ± 0.05 (Yemmireddy et al., 2013). Additionally, fluidized bed, air-impingement, and forced air dryers showed increasing drying times in this order. The lamina airflow condition and slow air flow rate (0.4m/s) were identified as the reason for the higher drying time recorded with a forced-air dryer (Yemmireddy et al., 2013).
4. Osmo-Convective Dehydration Technology
As alluded to in the introduction, osmo-convective dehydration combines the benefits of both osmotic dehydration and convective drying. This technology utilizes osmotic dehydration as a pretreatment step prior to convective drying. The osmotic dehydration step is the key to the peculiar benefit of this technology. Depending on the types of fruits the osmotic steps can be preceded with some fruit preparation steps such as washing, peeling, coring, and cutting into shapes. If the fruit has a tougher skin, for example, blueberries may first be scarified or frozen in order to rupture its skin and facilitate better mass transfer during osmotic dehydration step. Additionally, the osmotic dehydration step may be followed with slight rinsing of the osmosed fruits to remove excess solute attached to the fruits from the OD step.
4.1 Osmo-convective dehydration for fruits preservation
One advantage of osmotic-convective dehydration in fruit processing is its use as a hurdle technology to reduce the bacterial load and to ensure the final product’s safety. Dried fruits are considered safe from microbial hazards due to low water activity levels. Typically, water activity levels in the dried fruits are within 0.1 – 0.55 (Koyuncu et al., 2007; Yemmireddy et al., 2013; Akharume et al., 2016). Generally, no bacterial growth occurs below 0.61 water activity (Beuchat, 1981). However, it may be possible some food spoilage organisms that have contacted the dried fruit during handling may cause problems upon rehydration of the product (Sagar & Kumar, 2010). In a study, a selected number of commercial commodities sold at a retail market had indigenous yeast and mold bioburden counts ranging from 102 – 103 CFU/g; for example, an average of about 6061, 608, 264 CFU/g was found in walnuts, dates, and raisins (Ic et al., 2007). Osmotic dehydration in combination with drying may further increase the shelf life of the dried product by the impregnation of humectant will can easily lower the water activity of the fruit without necessarily reduce the moisture content. In a study that compared dried apple slices, Akharume et al., (2017) showed that the infusion of refined liquid smoke in the osmotic dehydration step prior to convective drying resulted in an extended lag phase in bacterial growth before any bacterial was detected compared to dried fruit without osmotic dehydration pretreatment that showed bacterial growth after three days. Other authors have reported that the shelf life of fresh-cut apple slices increased from 3-4 days to 6 days following osmotic dehydration (Castelló et al., 2009). Similarly, OD extends the shelf life of fresh strawberries to 7 days, which was further enhanced to 15 and 30 days when steam blanching or microwave treatments respectively was used before OD (Moreno et al., 2000).
4.2 Osmo-convective dehydration for fruits quality enhancement
Dried fruit’s color is a function of the chemical, biological and physical reactions that occur during thermal processing. Color correlates well with other physiochemical properties and it has been used as the fastest and the simplest indirect measurement of quality attributes (flavor and other pigments) (Pathare et al., 2013). Textural parameters of fruits are perceived with the sense of touch, either when the product is picked up by hand or placed in the mouth and chewed (Barrett et al., 2010). Quality attributes of fruits can be measured subjectively by visual or other sense organs, in most cases by a trained panelist. These attributes could also be measured objectively using instruments like texture analyzer, colorimeter etc. (Pathare et al., 2013). In a study on quality attributes of dried apples (untreated, sucrose osmosed and trehalose osmosed) stored over time, the group discovered that there was a high deviation in color parameter just after drying for all samples. The total color change of samples dried at 60℃ was 24.03; lower changes were observed in osmosed samples (14.24 – 17.14) (Aktas et al., 2013). During storage, degrees of lightness and hue increased with an increase in water activity, especially in the last 6 months. After 12 months, browning was observed in all samples and part of the untreated samples was starting to deteriorate. Samples osmosed in the 50 °Brix sucrose solution retained the color more than those osmosed in the 20 °Brix sucrose solution. Additionally, samples osmosed in both 20 and 50 °Brix trehalose solutions showed better color retention.
4.2.1 Effects of osmo-convective drying on the quality and sensory attributes of dried fruits
The effects of osmotic dehydration on color, textural, and sensory properties of dried apple slices were investigated by Chauhan et al., (2011) prior to drying, apples were osmosed in glucose, fructose, sucrose, maltose, sorbitol, or honey. Their result showed that the osmotic agent used had a significant effect (p < 0.05) on the color of the fruits. L* values were highest (87.75) for maltose osmosed samples and lowest (52.45) for honey osmosed samples. It was found that hardness was significantly (p<0.05) higher (20.104 N) in sucrose-dipped samples and at a minimum (4.441 N) in sorbitol-dipped samples. “Fracturability” and adhesiveness were significantly (p<0.05) higher in sorbitol (0.911 N) and honey (4.593 Ns)-osmosed samples, respectively. Springiness and cohesiveness (0.177, 0.151) were found to be at a maximum in fructose-treated samples, whereas chewiness (0.303N) was shown to be of high magnitude in sucrose-treated samples (Chauhan et al., 2011). Additionally, sensory evaluation of Osmo-dried apple slices revealed that sucrose-treated slices had significantly (p<0.05) more overall acceptability than other samples. Honey-treated samples showed significantly (p<0.05) lower color and texture values when compared to other samples as they possessed brown color and leathery texture. The samples osmosed with sucrose showed significantly (p<0.05) higher values for taste. The sorbitol osmosed samples had comparatively lower values for aroma, which was found to be the maximum in the case of fructose- and sucrose-dipped samples. The samples osmosed with maltose, sorbitol, or honey showed lower values for all the sensory parameters (Chauhan et al., 2011). In another experiment, Konopacka et al. (2008) compared sensory characteristics of the sour cherry in several osmotic solutions (concentrated apple juice (AJ), a mixture of concentrated apple juice and sour cherry juices (AJ+SCJ), de-acidified concentrated apple juice (DeAAJ) and sucrose solution as control (S)). They reported that the use of concentrated apple juices (AJ, AJ+SCJ) intensified the cherry taste and aroma, adjusting the flavor to consumer preference. The products resulting from AJ, and AJ+SCJ osmotic dehydration were perceived to be significantly more acidic but less sweet than those resulting from sucrose (S) or deacidified apple juice (DeAAJ) (Konopacka et al., 2008). Therefore, the choice of osmotic agent and its concentration, the temperature of the osmotic solution, and the fruit-to-solution ratio during osmotic dehydration have a large impact on product texture, color, and sensory quality (Pan et al., 2003).
4.2.2 Effects of osmo-convective drying on nutrients of dried fruits
Osmotic dehydration as a pretreatment to convective drying is been constantly explored to improve the quality of products. Though osmotic dehydration is associated with leaching out of the negligible amount of nutrients such as polyphenols and vitamin C, the loss can be complemented by careful choice and infusion of edible osmo-active agents that bind and retain these nutrients (such as trehalose) (Aktas et al., 2013; Raoult-Wack, 1994). To better understand the loss of vitamin C, apple slices were osmosed in either sucrose or trehalose at concentrations of 20 °Brix and 50 °Brix for 30 min then dried at 50, 60, and 70 ℃. The dried samples were then stored in a plastic bag at room temperature for 12 months. The loss of Vitamin C was the highest in the untreated sample compared to osmo-treated ones. Maximum vitamin C retention was recorded for the trehalose treatment. Again, vitamin C was higher in samples osmosed with 50 °Brix trehalose than that with 20 °Brix. All samples showed great loss of vitamin C during storage, especially the first 6 months (Aktas et al., 2013). Effects of additives in retention of vitamin C has been investigated during osmo-air-dried papaya (Germer et al., 2014): the treatment includes: CA/CL - 0.1 M citric acid with calcium lactate (0.5g/100g syrup); LA/CL - 0.1 M lactic acid with calcium lactate (0.5g/100g syrup); CA/CC - 0.1 M citric acid with calcium chloride (0.5g/100g syrup); LA/CC - 0.1 M lactic acid with calcium chloride (0.5g/100g syrup); PME/CC – PME (1mL/kg fruit) with calcium chloride (1g/kg fruit); and P - papaya without additives. The results showed a percentage of vitamin C retention of 33.1, 33.51, 46.62, 34.05, 62.81, and 56.78 for CA/CL, LA/CL, CA/CC, LA/CC, PME/CC, and P respectively.
Osmotic dehydration also affects polyphenol content. In an osmo-convective study, the retention of total phenol content was reported to be higher in osmosed samples than untreated samples (137.1mg/100g dry matter) after drying. However, at the end of 12-months’ storage, a higher phenol content was recorded for untreated samples than osmosed ones (Aktas et al., 2013). The polyphenols compounds are reported to leach out of apple slices during osmotic dehydration. In an experimental study involving two varieties of apples (Golden Delicious and Granny Smith), Kebe et al., (2014) demonstrated that the concentration of mannitol-based osmotic solutions (0, 0.2, 0.4, and 0.6M) was positively correlated with the amounts of leaching of polyphenols (Kebe et al., 2014). Likewise, loss of carotene from pumpkin and carrots has been correlated with water loss during osmotic dehydration (Pan et al., 2003). In another study, Giovanelli et al., (2013) reported on the effects of osmotic dehydration on the total phenolics, total anthocyanins and antioxidant activity on osmo-air drying of blueberries. Osmotic dehydration was carried out in either 60 °Brix sucrose solution or 48.6 °Brix glucose/fructose solution (1:1 w/w) and follow-up air drying was performed in a cabinet dryer set at 70 ℃, air velocity of 1.5 m/s. The report showed a significant decrease in total phenolics, total anthocyanins and antioxidant activity for all treatments, including untreated samples. Polyphenol degradation was the lowest in untreated samples, having a total phenolics, and total anthocyanins loss of 11 and 26%, respectively. The combined loss of 21-25% total phenolic was recorded for the blueberries osmosed in sucrose or glucose/fructose osmotic solutions. Likewise, losses of anthocyanins were 43% and 30%, reported respectively, for blueberries osmosed in sucrose and glucose/fructose solutions (Giovanelli et al., 2013).
4.3 Osmo-convective dehydration for new product development.
The peculiar advantage of infusing a selected solute into the fruit matrix during the osmotic dehydration step prior to drying is an area of ongoing research. The production of probiotic-dried fruits and other functional foods is one of the utilization of this advantage of osmo-convective dehydration technology. Betoret et al., (2003) developed a probiotic-enriched apple using osmotic dehydration under vacuum. Apples were impregnated with commercial apple juice containing Saccharomyces cerevisiae and with whole milk or apple juice containing 107 and 108 cfu/ml of Lactobacillus casei (ssp. rhamnosus). To assure stability and preservation, the fruit was further dried to a moisture content of 0.037 kg water/ kg dry matter and stored at room temperature for two months. L. casie viable cells in dried and stored products were greater than 106 cfu/g (Betoret et al., 2003). Very recently, Akharume et al., (2017) developed a dried-smoky-apple snack by infusing a smoky flavor into fresh-cut apples in an osmotic dehydration step followed by drying in a commercial convective dehydrator (Akharume et al., 2017; Akharume et al., 2019). The microbial assessment showed that total aerobic bacterial count was less than 105 cfu/g in the dried-smoky apples stored at room temperature from day 0 -5 months in both vacuum and non-vacuum packaging conditions. Other functional foods have also been produced. For example, β-carotene enriched apples (Santacruz-Vazquez et al., 2008), zinc enriched whole potato tuber (Erihemu et al., 2015), selenium-enriched cabbage (Haizhen et al., 2006), calcium and iron-fortified apples (Barrera et al., 2004). The ability to control the osmotic process conditions (the type of solution/osmo-active agents) step provides the versatility in the use of osmo-convective dehydration technology for new product development.
5. Future
The osmotic step in osmo-convective dehydration is a slow process owning to the thick epithelia cells and non-uniform cellular networks, which often create an obstruction to the two-way mass transfer of water and solute exchange within the fruit matrix. Efforts are being made to increase the micropores in the thick epithelia cells to facilitate the enhancement of mass transfer rates prior to performing OD. For example, treating fruits with ultra-sound, microwave, pulsed electric field, ohmic heating, centrifugal force or gamma-irradiation to rupture the skin have been tested and well-reviewed (Ahmed et al., 2016). In a study, apples exposed to microwave prior to drying were compared with freeze-drying and air-drying without pretreatment (Azarpazhooh & Ramaswamy, 2011). Microwave-assisted OD created a better texture, softer and chewable product, compared to that produced from the other two processes. In contrast, the freeze-dried product was brittle and air-dried was hard (Azarpazhooh & Ramaswamy, 2011). High pressure-assisted OD of apples (George et al., 2016) and ohmic heating assisted OD of apple cubes (Allali et al., 2009) have also been well reported.
With the dietary concern of using sugar solutions as osmo-active agent, the gray area of research will be in the development of new osmotic solutions that will increase nutrient retention and infuse bioactive compounds that will confer health benefits. Additionally, the use of osmosed fruit or osmo-convective dehydrated fruits as a vehicle for drug delivery in the gastrointestinal system is another is a gray area of research as well.
This work is partially supported by the USDA National Institute of Food and Agriculture, McIntire Stennis project 0222863 and 1007044. In addition, support was also provided by West Virginia University (WVU) Public Service Grant Award# P-14-006, and WVU Extension Service-Families and Health Programs. Partial support for this work was provided by the National Science Foundation (NSF)'s ADVANCE IT Program under Award HRD-1007978 as well as Northeast Center to Advance Food Safety- Special Projects Grant Program. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Ahmed, I., Qazi, I. M., & Jamal, S. (2016). Developments in osmotic dehydration technique for the preservation of fruits and vegetables. Innovative Food Science & Emerging Technologies, 34, 29-43.
View ArticleAhvenainen, R. (1996). New approaches in improving the shelf life of minimally processed fruit and vegetables. Trends in Food Science & Technology, 7(6), 179-187. 10022-4
View ArticleAkharume, F., Singh, K., Jaczynski, J., & Sivanandan, L. (2017). Microbial shelf stability assessment of osmotically dehydrated smoky apples. LWT-Food Science and Technology.
View ArticleAkharume, F., Singh, K., & Sivanandan, L. (2019). Effects of liquid smoke infusion on osmotic dehydration kinetics and microstructural characteristics of apple cubes. Journal of Food Engineering, 246, 51-57.
View ArticleAkharume, F. U., Singh, K., & Sivanandan, L. (2016). Characteristics of apple juice and sugar infused fresh and frozen blueberries. LWT - Food Science and Technology, 73, 448-457.
View ArticleAktas, T., Ulger, P., Daglioglu, F., & Hasturk, F. (2013). Changes of nutritional and physical quality characteristics during storage of osmotic pretreated apple before hot air drying and sensory evaluation. Journal of Food Quality, 36(6), 411-425.
View ArticleAllali, H., Marchal, L., & Vorobiev, E. (2009). Effect of blanching by ohmic heating on the osmotic dehydration behavior of apple cubes. Drying Technology, 27(6), 739-746.
View ArticleAllali, H., Marchal, L., & Vorobiev, E. (2010). Effects of vacuum impregnation and ohmic heating with citric acid on the behaviour of osmotic dehydration and structural changes of apple fruit. Biosystems Engineering, 106(1), 6-13.
View ArticleAssis, F. R., Morais, R., & Morais, A. M. (2016). Mathematical modelling of osmotic dehydration kinetics of apple cubes. Journal of Food Processing and Preservation.
View ArticleAzarpazhooh, E., & Ramaswamy, H. S. (2011). Optimization of microwave-osmotic pretreatment of apples with subsequent air-drying for preparing high-quality dried product. International Journal of Microwave Science and Technology, 2011.
View ArticleAzoubel, P. M., & Elizabeth Xidieh Murr, F. (2004). Mass transfer kinetics of osmotic dehydration of cherry tomato. Journal of Food Engineering, 61(3), 291-295. 00132-8
View ArticleAzuara, E., Cortés, R., Garcia, H. s., & Beristain, C. i. (1992). Kinetic model for osmotic dehydration and its relationship with fick's second law. International journal of food science & technology, 27(4), 409-418.
View ArticleBarrera, C., Betoret, N., & Fito, P. (2004). Ca2+ and fe2+ influence on the osmotic dehydration kinetics of apple slices (var. Granny smith). Journal of Food Engineering, 65(1), 9-14.
View ArticleBetoret, N., Puente, L., Dı́az, M. J., Pagán, M. J., Garcı́a, M. J., Gras, M. L., . . . Fito, P. (2003). Development of probiotic-enriched dried fruits by vacuum impregnation. Journal of Food Engineering, 56(2-3), 273-277. 00268-6
View ArticleBeuchat, L. R. (1981). Microbial stability as affected by water activity. Cereal Foods World, 26(7), 345-349.
Blanchette, L., & Brug, J. (2005). Determinants of fruit and vegetable consumption among 6-12-year-old children and effective interventions to increase consumption. Journal of Human Nutrition and Dietetics, 18(6), 431-443. doi:10.1111/j.1365-277X.2005.00648.x PMid:16351702
View Article PubMed/NCBICastelló, M., Igual, M., Fito, P., & Chiralt, A. (2009). Influence of osmotic dehydration on texture, respiration and microbial stability of apple slices (var. Granny smith). Journal of Food Engineering, 91(1), 1-9.
View ArticleChauhan, O. P., Singh, A., Singh, A., Raju, P. S., & Bawa, A. S. (2011). Effects of osmotic agents on colour, textural, structural, thermal, and sensory properties of apple slices. International Journal of Food Properties, 14(5), 1037-1048. doi:10.1080/10942910903580884
View ArticleChiralt, A., & Talens, P. (2005). Physical and chemical changes induced by osmotic dehydration in plant tissues. Journal of Food Engineering, 67(1-2), 167-177.
View ArticleCorrêa, J. L. G., Pereira, L. M., Vieira, G. S., & Hubinger, M. D. (2010). Mass transfer kinetics of pulsed vacuum osmotic dehydration of guavas. Journal of Food Engineering, 96(4), 498-504.
View ArticleCrank, J. (1975). The mathematics of diffusion: Oxford: Clarendon Press.
Erihemu, Hironaka, K., Koaze, H., Oda, Y., & Shimada, K. (2015). Zinc enrichment of whole potato tuber by vacuum impregnation. Journal of Food Science and Technology, 52(4), 2352-2358. doi:10.1007/s13197-013-1194-5 PMid:25829619
View Article PubMed/NCBIFAO. (2019). Crop statistics Retrieved 10/7/2019, from Food and Agriculture Organization of the United Nations (FAO)
View ArticleFick, A. (1855). V. On liquid diffusion. The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science, 10(63), 30-39.
View ArticleFito, P. (1994). Modelling of vacuum osmotic dehydration of food. Journal of Food Engineering, 22(1), 313-328. 90037-X
View ArticleFito, P., & Pastor, R. (1994). Non-diffusional mechanisms occurring during vacuum osmotic dehydration. Journal of Food Engineering, 21(4), 513-519. 90070-1
View ArticleGeankoplis, C. (1993). Transport processes and unit operations. Prentice-hall. New Jersey, USA.
George, J. M., Senthamizh Selvan, T., & Rastogi, N. K. (2016). High-pressure-assisted infusion of bioactive compounds in apple slices. Innovative Food Science & Emerging Technologies, 33, 100-107.
View ArticleGermer, S. P. M., Ferrari, C. C., Lancha, J. P., Berbari, S. A. G., Carmello-Guerreiro, S. M., & Ruffi, C. R. G. (2014). Influence of processing additives on the quality and stability of dried papaya obtained by osmotic dehydration and conventional air drying. Drying Technology, 32(16), 1956-1969. doi:10.1080/07373937.2014.924963
View ArticleGiovanelli, G., Brambilla, A., & Sinelli, N. (2013). Effects of osmo-air dehydration treatments on chemical, antioxidant and morphological characteristics of blueberries. LWT-Food Science and Technology, 54(2), 577-584.
View ArticleGiraldo, G., Talens, P., Fito, P., & Chiralt, A. (2003). Influence of sucrose solution concentration on kinetics and yield during osmotic dehydration of mango. Journal of Food Engineering, 58(1), 33-43. 00331-X
View ArticleHaizhen, M., Min, Z., & Jincai, S. (2006). Effect of drying process parameters on dehydrated cabbage enriched with selenium. Drying Technology, 24(12), 1657-1663. doi:10.1080/07373930601031497
View ArticleIc, E., Kottapalli, B., Maxim, J., & Pillai, S. D. (2007). Electron beam radiation of dried fruits and nuts to reduce yeast and mold bioburden. Journal of Food Protection®, 70(4), 981-985. PMid:17477270
View Article PubMed/NCBIKebe, M., Renard, C., Amani, G., & Maingonnat, J.-F. (2014). Kinetics of apple polyphenol diffusion in solutions with different osmotic strengths. Journal of Agricultural and Food Chemistry, 62(40), 9841-9847. PMid:25213754
View Article PubMed/NCBIKhan, M. A., Shukla, R., & Zaidi, S. (2011). Mass transfer during osmotic dehydration of apple using sucrose, fructose and maltodextrin solution. Paper presented at the Published in the Proceedings of 11th International Congress on Engineering and Food-Athens, Greece.
Khan, M. R. (2012). Osmotic dehydration technique for fruits preservation-a review. Pakistan Journal of Food Sciences, 22(2), 71-85.
Konopacka, D., Jesionkowska, K., Mieszczakowska, M., & Płocharski, W. (2008). The usefulness of natural concentrated fruit juice as osmotic agent for osmo-dehydrated dried fruit production. Journal of Fruit and Ornamental Plant Research, 16, 275-284.
Lazarides, H. N., Katsanidis, E., & Nickolaidis, A. (1995). Mass transfer kinetics during osmotic preconcentration aiming at minimal solid uptake. Journal of Food Engineering, 25(2), 151-166. 00006-U
View ArticleLe Maguer, M., & Yao, Z. (1995). Mass transfer during osmotic dehydration at the cellular level. Food Preservation by Moisture Control: Fundamentals and Applications, 325-350.
Lewicki, P. P., & Lenart, A. (1995). 28 osmotic dehydration of fruits and vegetables.
Maguer, M. L. (1997). Mass transfer modeling in structured foods. Food Engineering 2000, 253-269.
View ArticleMarcotte, M., & Le Maguer, M. (1992). Mass transfer in cellular tissues. Part ii: Computer simulations vs experimental data. Journal of Food Engineering, 17(3), 177-199. 90068-H
View ArticleMauro, M. A., Dellarosa, N., Tylewicz, U., Tappi, S., Laghi, L., Rocculi, P., & Rosa, M. D. (2016). Calcium and ascorbic acid affect cellular structure and water mobility in apple tissue during osmotic dehydration in sucrose solutions. Food Chemistry, 195, 19-28. PMid:26575708
View Article PubMed/NCBIMcNamara, P. E., Ranney, C. K., Kantor, L. S., & Krebs-Smith, S. M. (1999). The gap between food intakes and the pyramid recommendations: Measurement and food system ramifications. Food Policy, 24(2), 117-133. doi: 00020-2 00020-2
View ArticleMoreno, J., Chiralt, A., Escriche, I., & Serra, J. A. (2000). Effect of blanching/osmotic dehydration combined methods on quality and stability of minimally processed strawberries. Food Research International, 33(7), 609-616. 00097-1
View ArticleNsonzi, F., & Ramaswamy, H. (1998). Osmotic dehydration kinetics of blueberries. Drying Technology, 16(3-5), 725-741.
View ArticleOchoa-Martinez, C. I., Ramaswamy, H. S., & Ayala-Aponte, A. A. (2007). A comparison of some mathematical models used for the prediction of mass transfer kinetics in osmotic dehydration of fruits. Drying Technology, 25(10), 1613-1620. doi:10.1080/07373930701590665
View ArticlePan, Y., Zhao, L., Zhang, Y., Chen, G., & Mujumdar, A. S. (2003). Osmotic dehydration pretreatment in drying of fruits and vegetables. Drying Technology, 21(6), 1101-1114.
View ArticlePanagiotou, N. M., Karathanos, V. T., & Maroulis, Z. B. (1998). Mass transfer modelling of the osmotic dehydration of some fruits. International Journal of Food Science & Technology, 33(3), 267-284. doi:10.1046/j.1365-2621.1998.00167.x
View ArticlePathare, P. B., Opara, U. L., & Al-Said, F. A.-J. (2013). Colour measurement and analysis in fresh and processed foods: A review. Food and Bioprocess Technology, 6(1), 36-60.
View ArticlePeleg, M. (1988). An empirical model for the description of moisture sorption curves. Journal of Food Science, 53(4), 1216-1217.
View ArticleRamaswamy, H., & Nsonzi, F. (1998). Convective-air drying kinetics of osmotically pre-treated blueberries. Drying Technology, 16(3-5), 743-759.
View ArticleRaoult-Wack, A.-L., Guilbert, S., Maguer, M. L., & Rios, G. (1991). Simultaneous water and solute transport in shrinking media - part 1. Drying Technology, 9(3), 589-612. doi:10.1080/07373939108916698
View ArticleRaoult-Wack, A. L. (1994). Recent advances in the osmotic dehydration of foods. Trends in Food Science & Technology, 5(8), 255-260. 90018-3
View ArticleRastogi, N., Angersbach, A., & Knorr, D. (2000). Evaluation of mass transfer mechanisms during osmotic treatment of plant materials. Journal of Food Science-Chicago-, 65(6), 1016-1021.
View ArticleRastogi, N. K., Raghavarao, K. S. M. S., Niranjan, K., & Knorr, D. (2002). Recent developments in osmotic dehydration: Methods to enhance mass transfer. Trends in Food Science & Technology, 13(2), 48-59. 00032-8
View ArticleRatti, C. (2001). Hot air and freeze-drying of high-value foods: A review. Journal of Food Engineering, 49(4), 311-319. 00228-4
View ArticleRodrigues, A. E., & Mauro, M. A. (2008). Effective diffusion coefficients behavior in osmotic dehydration of apple slices considering shrinking and local concentration dependence. Journal of Food Process Engineering, 31(2), 207-228.
View ArticleSabarez, H. T. (2016). 14 - airborne ultrasound for convective drying intensification. In K. Knoerzer, P. Juliano, & G. Smithers (Eds.), Innovative food processing technologies (pp. 361-386): Woodhead Publishing.
View ArticleSacchetti, G., Gianotti, A., & Dalla Rosa, M. (2001). Sucrose-salt combined effects on mass transfer kinetics and product acceptability. Study on apple osmotic treatments. Journal of Food Engineering, 49(2-3), 163-173. 00206-5
View ArticleSagar, V., & Kumar, P. S. (2010). Recent advances in drying and dehydration of fruits and vegetables: A review. Journal of Food Science and Technology, 47(1), 15-26. PMid:23572596
View Article PubMed/NCBISalvatori, D., AndrÉS, A., Albors, A., Chiralt, A., & Fito, P. (1998). Structural and compositional profiles in osmotically dehydrated apple. Journal of Food Science, 63(4), 606-610. doi:10.1111/j.1365-2621.1998.tb15795.x
View ArticleSantacruz-Vázquez, C., Santacruz-Vázquez, V., Jaramillo-Flores, M. E., Chanona-Pérez, J., Welti-Chanes, J., & Gutiérrez-López, G. F. (2008). Application of osmotic dehydration processes to produce apple slices enriched with β-carotene. Drying Technology, 26(10), 1265-1271. doi:10.1080/07373930802307266
View ArticleShi, J., & Le Maguer, M. (2002). Osmotic dehydration of foods: Mass transfer and modeling aspects. Food Reviews International, 18(4), 305-335. doi:10.1081/FRI-120016208
View ArticleShi, J., Pan, Z., McHugh, T. H., & Hirschberg, E. (2009). Effect of infusion method and parameters on solid gain in blueberries. Food and Bioprocess Technology, 2(3), 271-278. doi:10.1007/s11947-008-0116-4
View ArticleSingh, B., Kumar, A., & Gupta, A. K. (2007). Study of mass transfer kinetics and effective diffusivity during osmotic dehydration of carrot cubes. Journal of Food Engineering, 79(2), 471-480.
View ArticleSingh, B., Panesar, P. S., & Nanda, V. (2008). Osmotic dehydration kinetics of carrot cubes in sodium chloride solution. International Journal of Food Science & Technology, 43(8), 1361-1370. doi:10.1111/j.1365-2621.2007.01623.x
View ArticleSosa, N., Salvatori, D. M., & Schebor, C. (2012). Physico-chemical and mechanical properties of apple disks subjected to osmotic dehydration and different drying methods. Food and Bioprocess Technology, 5(5), 1790-1802. doi:10.1007/s11947-010-0468-4
View ArticleTiwari, R. (2005). Application of osmo-air dehydration for processing of tropical fruits in rural areas. Indian food industry, 24(6), 62-69.
Torreggiani, D. (1993). Osmotic dehydration in fruit and vegetable processing. Food Research International, 26(1), 59-68. 90106-S
View ArticleUSDANASS. (2017). Crop production (1936-3737).
U.S. Department of Agriculture and U.S. Department of Health and Human Services. (2019). Dietary guidelines for Americans, 2015-2020 (8th ed., p. 38-39). Washington, DC: U.S. Government Printing Office.
van Nieuwenhuijzen, N. H., Zareifard, M. R., & Ramaswamy, H. S. (2001). Osmotic drying kinetics of cylindrical apple slices of different sizes. Drying Technology, 19(3-4), 525-545. doi:10.1081/DRT-100103932
View ArticleVega-Gálvez, A. A., Lemus-Mondaca, R. R., Tello-Ireland, C. C., Miranda, M. M., & Yagnam, F. F. (2009). Kinetic study of convective drying of blueberry variety o'neil (vaccinium corymbosum l.).
View ArticleWang, Z., Wei, T., & Zhang, M. (2015). Effects of vacuum and normal pressure impregnation on water loss and solid gain of apple (malus pumila mill). Journal of Food Processing and Preservation, 39(6), 1045-1050.
View ArticleYadav, A. K., & Singh, S. V. (2014). Osmotic dehydration of fruits and vegetables: A review. Journal of Food Science and Technology, 51(9), 1654-1673. PMid:25190823
View Article PubMed/NCBIYemmireddy, V. K., Chinnan, M. S., Kerr, W. L., & Hung, Y.-C. (2013). Effect of drying method on drying time and physico-chemical properties of dried rabbiteye blueberries. LWT-Food Science and Technology, 50(2), 739-745.
View ArticleYu, L. (1998). Osmotic-air dehydration of cherries and blueberries. unpublished thesis. University of Manitoba, Winnipeg Manitoba. University of Manitoba, Winnipeg Manitoba. Retrieved from
View ArticleZhang, M., Chen, H., Mujumdar, A. S., Tang, J., Miao, S., & Wang, Y. (2017). Recent developments in high-quality drying of vegetables, fruits, and aquatic products. Critical Reviews in Food Science and Nutrition, 57(6), 1239-1255. doi:10.1080/10408398.2014.979280 PMid:26055086
View Article PubMed/NCBI