Xiulian Ren
School of Marine Science and Technology, Harbin Institute of Technology at Weihai, Weihai Wenhua West Road 2, Weihai, Shandong 264209, China
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 4 ISSUE: 8
Page No: 934-945
Xiulian Ren
School of Marine Science and Technology, Harbin Institute of Technology at Weihai, Weihai Wenhua West Road 2, Weihai, Shandong 264209, China
Yilong Ma(yilong.ma@hfut.edu.cn)
Min Wang(wangmin20050606@163.com)
Alessandro Pellizzaro(pellizzaroa@acquedelchiampospa.it)
Jaros?aw L Przyby?(jaroslaw_przybyl@sggw.pl)
Xiulian Ren, Recovery and separation of lactic and malic acids from fermentation broth with N,N,N,N-Tetrabutylbutanediamide by reactive liquid−liquid extraction and stripping(2019) Journal of Food Science & Technology 4(8)
It is of great importance to extract and separate lactic and malic acids which used as raw materials in chemical industry. Taking into account its desirable selectivity of lactic and malic acids, the discovery of the novel extractant called N,N,N,N-Tetrabutylbutanediamide (N4423) is a innovation in this paper. Separation and purification the malic and lactic acids that present in the fermentation broth by reactive liquid-liquid extraction is the principal objective of this study.
The lactic acid extraction was ideal when the reaction performed in the optimum conditions: 60 wt% of N4423, phase ratio (O/A) of 3, temperature at 35℃ and magnetic stirred 5 minutes. As a result, the purity of lactic acid exceeded 98%. Based on the slope method, the equilibrium concentration of lactic and malic acids in the organic phase were high enough to form the complexes which the mole ratio of N4423 to lactic acid and malic acid were 1.61, 1.86 respectively. FT-IR characterization indicated that the formation of hydrogen bonding between N4423 and lactic acid were predominant to the excellent extraction efficiency.
The adopted reactive liquid-liquid extraction method is of low cost, low energy consumption and environmental friendliness. The N4423 has good extraction and separation efficiency of lactic and malic acids, the obtained lactic acid has high purity. The results gathered in this work might prove to be a potential approach to acquire lactic and malic acids.
Keywords: reactive liquid−liquid extraction; separation; purification; lactic acid; malic acid
Lactic acid is an important platform chemical widely used in food1 (e.g. candy, beverages, bakery, meat), pharmaceutical2 (e.g. dialysisand disinfection), cosmetics (e.g. moisturizers, anti-acne), as well as an intermediate for the composite of commercial chemicals.1, 3 Malic acid is also seen as a potential substitute for petroleum-derived chemical maleic anhydride.4 Recently, in pharmaceutical and textile industry, the demand of lactic acid used in the production of biopolymer polylactic acid (PLA, a biodegradable polymer) has increased dramatically.5 Nevertheless, the most challenge in the production of lactic and malic acids from fermentation broth is to design an efficient extraction and separation process.
Conventionally, many studies are implemented for separation of the lactic acid, including membrane separation,6-9 adsorption,10 ion exchange,11 direct distillation,12 reactive distillation,13 molecular distillation,14 reactive extraction etc.15-17 Membrane separation can be used to avoid toxicity, but it is difficult to clean and sterilize, which would limit its application. Adsorbents are used to adsorb carboxylic acids and are easily desorbed with alcohol eluents, but it is hard to separate lactic acid from alcohol. Ion exchange process requires the regeneration of ion-exchange resin, the whole process is complicated and the resin utilization rate is low. Dimers with high boiling point can be formed during direct distillation, and the energy consumption is very high. Reactive distillation leads to the formation of by-product, thus increasing the purification cost. Molecular distillation is a burgeoning separation technology, which can obtain high-purity lactic acid, but high investment should be involved in the early stage. However, the reactive liquid-liquid extraction shows advantages like high yield and high conversion of substrate, it can not only easily extract organic acids from fermentation broth to prevent pH value from decreasing but also can re-extract the organic acids and the used extractant can be recovered.
The most commonly used organic solvents to extract organic acids from wastewater solution are tri-n-butylphosphate (TBP), tri-n-octylamine (TOA) and tri-n-octylphosphineoxide (TOPO), all of which can be used pure or as a mixture with other organic solvents such as hexanol, octanol, decanol, toluene, and dodecane. Matsumoto et al. obtained poor results, with partition coefficients close to one, using mixtures of TOA and/or TBP in hexane.18, 19 Also, Hossain et al. reported distribution coefficients lower than one for extractions of lactic acid using pure solvents, like decanol, dodecane, and hexane, without an extractant.20 However, the synthesis and corresponding post-treatment process of many traditional organic solvents are complicated and would bring up some additional environmental hazards such as toxicity, volatility, and flammability.21-24 Thereby, the development of new, less hazardous solvents has been receiving increased attention.25 Amide extractants that are thought as potential replacements for traditional organic compounds play a central role. Moreover, amide extractants have many advantages, such as their physical/chemical properties which can be finely adjusted by a careful selection of lactic acid, high purity lactic acid that can be obtained by extraction. In addition, the hydrolysis resistance and radiolysis performance of amide extractants are comparable to those of TBP, but the degradation products do not affect the extraction process. In fact, reactive liquid-liquid extraction with a specified extractant giving a higher distribution coefficient has been proposed as a promising technique for the recovery of carboxylic and hydroxycarboxylic acids.26, 27
2.1. Materials
Lactic acid fermentation broth was provided by Henan Jindan Lactic Technology Co., Ltd. (Henan, China). The diluent solvent was 3# white oil, the polar diluent was C10-12 (mixture of decanol, undecyl alcohol, dodecanol). Aqueous D-lactic acid (LA, CAS 10326-41-7), with mass percentage purity between 69.0% and 71.0%. Solid L-(-)-malic acid (MA, CAS 97-67-6), with mass percentage purity higher than 99.0%. The other chemicals used in this paper were Hydrochloric acid (HCl, AR, ≥36%), Sodium hydroxide (NaOH, AR, ≥96%), and Sodium carbonate (Na2CO3, AR, ≥99%). All chemicals were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). All extractants if not declared were synthesized by laboratory staffs.
2.2. Apparatus
Quantitative analysis of lactic and malic acids were performed on an Agilent 1200 series HPLC system. The chromatographic separation was carried out on a ZORBAX 300SB-C18 column (4.6 mm×250 mm, 5 µm), the UV light detection wavelength was 210 nm. The mobile phase was 0.005 M sodium dihydrogen phosphate aqueous solution with a flow rate of 0.6 ml/min. The detection temperature of column was kept as 30℃ and the total testing time was 30 minutes. The calibration of the method was carried out using internal standards and by analyzing the samples with known amount of each acid. A calibration curve was established for each acid under study and was used to calculate the concentration of each acid in aqueous phase. The corresponding acid concentration in the organic phase was calculated by mass balance. The pH of the aqueous phase before and after extraction was measured with a pH-meter that equipped with the WTW microprocessor and a temperature compensating probe. All experiments were performed for three times, and the relative deviations were found to be within 1.5%. FT-IR characterization was performed on a fourier transform infrared spectrophotometry (NICOLET 380, Thermo Electron corporation, USA) and the scanning wavenumber ranged from 4000 cm-1 to 500 cm-1.
2.3. Experimental procedure
In reactive liquid-liquid extraction, 10 g of aqueous (containing known concentrations of lactic acid and malic acids) was mixed with 30 g of organic phase (containing 40 wt% 3# white oil and 60 wt% N4423) in a 250 mL erlenmeyer. A magnetic water bath stirring was added, and the mixture was stirred for 5 minutes at 1000 rpm and the temperature was 35°C. After that, standing still 5 minutes was considered sufficient to separate the two phases after extraction. To determine the lactic acid concentration, the aqueous phase was titrated with NaOH (0.05 M) using phenolphthalein as the indicator.28 The amount of lactic acid in the organic phase was determined by a mass balance taking into account the volumes of the organic and aqueous phases respectively. The approximate method has been used in the literature.29, 30 For more accurate determination, the HPLC should be used.31, 32
In back-extraction, double distilled water was added to recover organic acids in a 3:1 (O/A) ratio, the mixture was then stirred and kept under vigorous stirring for at least 5 minutes at 70℃ so that equilibrium was attained.
Under the same conditions, each sample was analyzed three times and the average was reported. The analysis is carried out immediately after phase separation in order to avoid acid which may be oxidized by air, evaporated, or eventually degraded by bacteria. The experimental error is less than 5%. This average is used for graphical representation.
2.4. Definition of the characteristic parameters
According to the concentration of lactic or malic acid, the extraction efficiency is defined by percentage of extraction (E(%)), the distribution coefficient (D(E)) is defined as the ratio of lactic or malic acid concentration in organic phase (C org ) to that in the aqueous phase (C aq ), which can be calculated by the following equation.
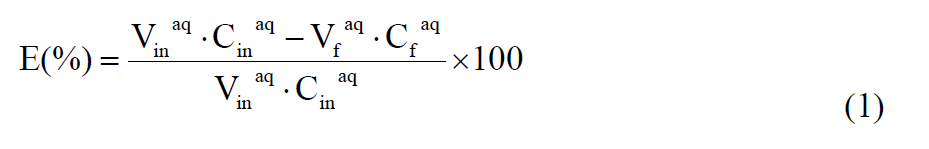

The organic reaction complex (BHLA)org when put in contact with deionized water, with positive and negative ions, it would result in the following reaction:
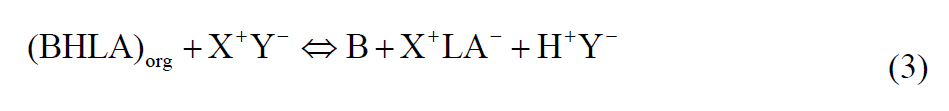


where D(S) and BE(%) are the distribution coefficient and stripping percentage of the back-extraction process, respectively.
The functional analysis of the ratio of the distribution coefficients of different acids define an favorable conditions for acids separation, which called separation
factor (β) and the equation indicated as follows:

Where DHLA and DH2MA are the distribution coefficients of lactic and malic acids, respectively.
2.5. Equilibrium and mechanism of extraction analysis
The extraction equilibrium of lactic acid (HA) extracted by extractant (B) could be demonstrated that there was a set of equilibria which involved the formation of complex (HA-nB). The reaction could be represented as follows:

It is usually assumed that the activities of the species in the organic phase are proportional to the concentrations of those species, and that the proportionality constants are included in the equilibrium constant. The activity of lactic acid is assumed to be proportional to the equilibrium concentration of undissociated acid in the aqueous phase. Thus, the apparent equilibrium constant (Kex) of overall reaction could be written as follows:

The distribution coefficient of lactic acid (D) is defined by:

The relationship between the equilibrium concentration of the extractant ( [B] ) and the distribution ratio of lactic acid (D) exhibited as follows:

In this process, the distribution ratio (D) is approximately linear with the extraction equilibrium constant, ie:

A linear relationship between the distribution coefficient (D) and 1/T could be described:
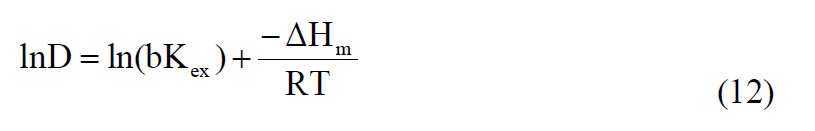
3.1. Extractant selection
Generally, the extraction extent of lactic acid significantly depend on the type of extraction system and reagent.33 At first, the lactic acid extracted by 3# white oil experimentally, which proved that the extraction percentage was less than 1% under the experimental conditions. So the 3# white oil was considered as diluent. After that, various 45 wt% extractants that proportionally mixd with 45 wt% 3# white oil and 10 wt% C10-12 were used to react with lactic acid fermentation broth. In this step, the objective was to choose the optimum extraction system that has the highest extraction degree in extracting lactic acid. The obtained results of the tests are graphically presented in Figure 1. From the results, four extractants have shown the better selectivity and the variation of their distribution coefficients versus the concentrations are consigned numerically in Table 1. Based on these tests, the N4423 has more desirable selectivity with respect to the lactic acid because of the greatest value of E(%). Meanwhile, the value of D(E) for N4423 are slightly higher than those of other extractants. Such results are confirmed by other works that follow.
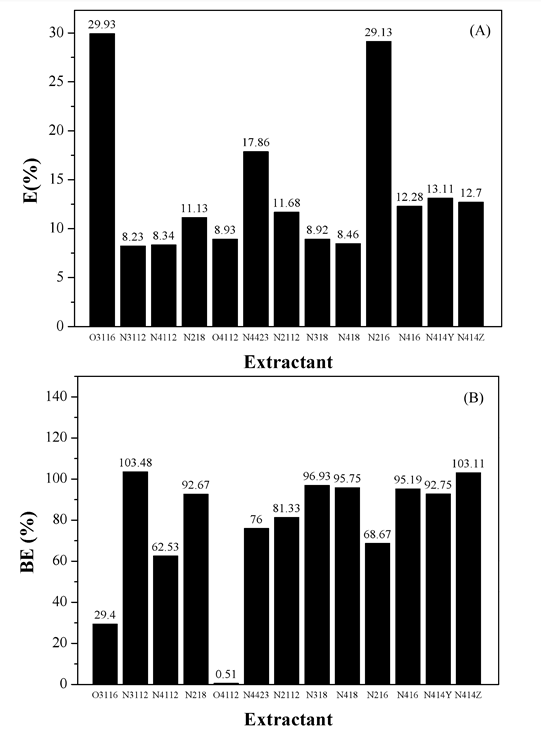
Figure 1. Extraction rate (A) and back extraction ratio (B) of lactic acid with various 45 wt% extractants that proportionally mixd with 45 wt% 3# white oil and 10 wt% C10-12.
Table 1. E (%), D(E), BE(%), D(S) of four extractants
|
Extractant |
E(%) |
D(E) |
BE(%) |
D(S) |
|
N2112 |
13.13 |
0.15 |
85.62 |
0.17 |
|
N3112 |
13.37 |
0.13 |
13.51 |
13.51 |
|
N4112 |
8.14 |
0.09 |
0.09 |
0.09 |
|
N4423 |
18.15 |
0.22 |
74.73 |
0.34 |
3.2. Influence of various parameters on lactic acid
The results of the lactic acid extraction process are presented as the distribution coefficient and the percentage of extraction efficiency.
3.2.1. Effect of temperature
Since the temperature significantly affects the transfer of complex or relevant molecules between aqueous and organic phase, the extraction experiments were performed at different temperature in order to acquire the ideal extraction efficiency. The outcome is displayed in Figure 2. It is so evident that with the extraction temperature increasing from 25℃ to 75℃, the concentrations of lactic acid in the organic phase decrease sharply. It could be inferred that the complexation reaction of lactic acid and N4423 in the organic phase involves proton transfer or hydrogen bond formation and are therefore expected to be exothermic. Also, formation of a complex makes the system more ordered and therefore decreases the entropy. Thus, as the temperature increased, the amount of extracted lactic acid decreased. So hypothermia is favorable for a better extraction, the temperature was selected as 35℃ for the continuous research.
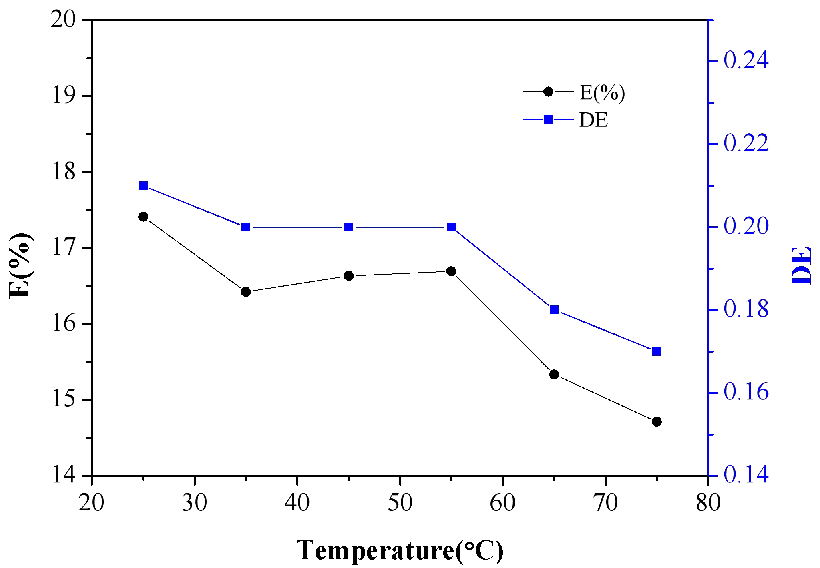
Figure 2. Effect of temperature on the extraction of lactic acid.
3.2.2. Effect of mass fraction of N4423
The effect of the mass fraction scale of N4423 is obvious. The total mass fraction of the organic phase was unchanged, however, the mass fraction of N4423 was varied as demonstrated in Figure 3. As the mass fraction of N4423 increased from 30 wt% to 70 wt%, the values of E(%) and D(E) increased and reached a maximum that were 26.5% and 0.375 respectively. At the beginning, the amount of lactic acid in the aqueous phase was sufficient relative to the organic phase, with the concentration of N4423 increasing, the probability of binding with unit lactic acid increased, thus, prompting the extraction reaction. However, as the concentration of N4423 went up continually, the growth of E(%) and D(E) gradually became slower, because the amount of lactic acid was insufficient relative to the organic phase when the concentration of N4423 increased to a certain extent. Naturally, the mass fraction was chosen as 60 wt%.
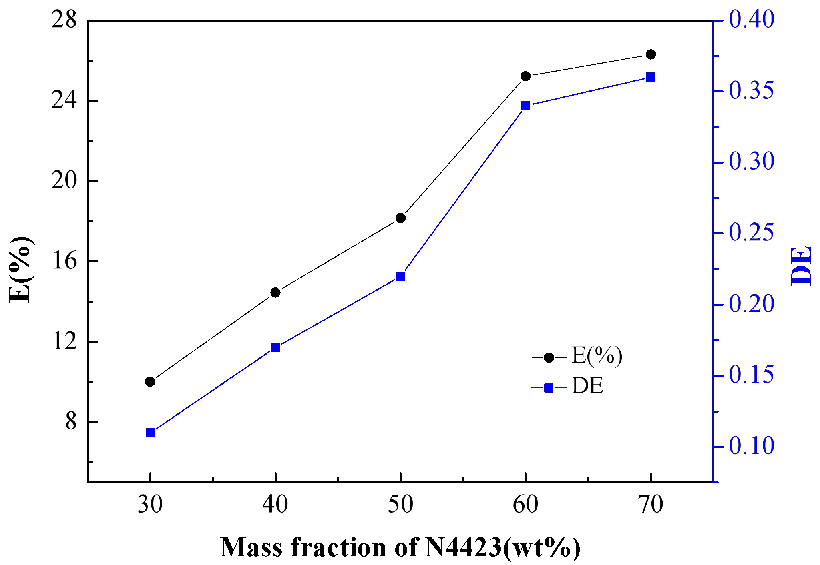
Figure 3. Effect of mass fraction of N4423 on the extraction of lactic acid.
3.2.3. Effect of mass fraction of C10-12
The diluents affect the basicity of the amine and thus the stability of the ion pair formed and its solvation. The stability of the complex governs the equilibrium conditions of acid extraction, especially at low loading ratio, where the equilibrium aqueous acid concentration is very low. Under such conditions, polar diluents are more favorable than the zero-polarity, low dielectric constant, aliphatic and aromatic hydrocarbons.34, 35 Therefore, it is necessary to explore the effect of the concentration of polar diluent (C10-12) on the extraction of lactic acid. Figure 4 shows the extraction extent as a function of the initial concentration of C10-12 in the range of 0 wt%-0.5 wt%. In general, C10-12 is used frequently in solvents to short extraction delamination time. It was experimentally found that the concentration of C10-12 affected negatively the uptake of lactic acid in the organic phase. It is deduced that a small amount of C10-12 might also weaken the solubility of the lactic acid-N4423 complex. So the low concentration of C10-12 is beneficial to a better distribution coefficient. Therefore, not to add C10-12 to the organic solvents for improving extraction rate.
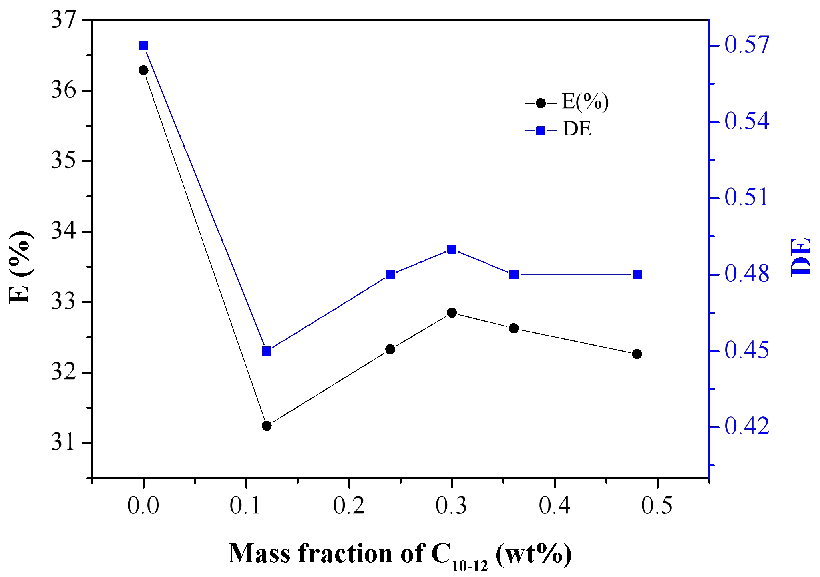
Figure 4. Effect of mass fraction of C10-12 on the extraction of lactic acid.
3.2.4. Effect of extraction time
The extraction time influences the mass transfer kinetics between the reaction of lactic acid and N4423 molecules. In order to determine when the complexation reaction could reach equilibrium, the experiments related extraction time were carried out. Taking into account the results presented in Figure 5, at the beginning of the extraction reaction, the values of E(%) and D(E) were low because N4423 and lactic acid fermentation liquid were not in perfect contact. As the extraction time lengthened, the mass transfer reached a certain level, the values of E(%) and D(E) increased and then reached the maximum in 5 minutes. However, as the reaction proceeded, the reducing of lactic acid concentration in aqueous phase decreased the mass transfer kinetics between lactic acid molecules, resulting in the decrease of the values of E(%) and D(E) slightly.
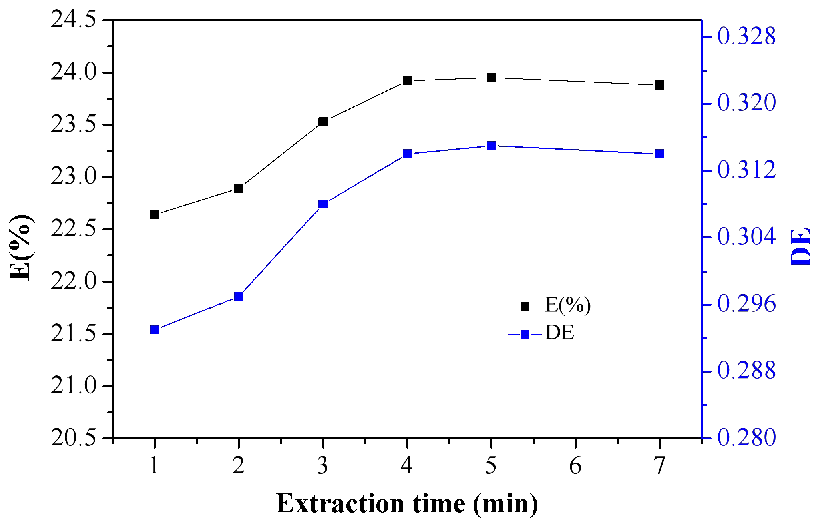
Figure 5. Effect of extraction time on extraction of lactic acid.
3.2.6. Effect of phase ratio (O/A)
Data corresponding to the value of O/A of lactic acid in the organic phase yields two straight lines with different slopes, as shown in Figure 6. It is clear that the increasing phase ratio leads at a progressive improvement of the values of E(%) and D(E) of lactic acid. When O/A=3.0 was applied for the extraction, 43% and 0.78 of the E(%) and D(E) could be gained, respectively.
The value of O/A is mainly concerned with the extraction time. In general, if the extraction rate is constant, the larger the value of O/A, the smaller the heat transfer resistance between N4423 and lactic acid molecules, and the shorter the extraction time. Therefore, the relatively higher phase ratio is favorable for the extraction of lactic acid. However, the extractant is wasted when the flow rate of the organic phase is too large, economically, the appropriate O/A=3 should be selected.
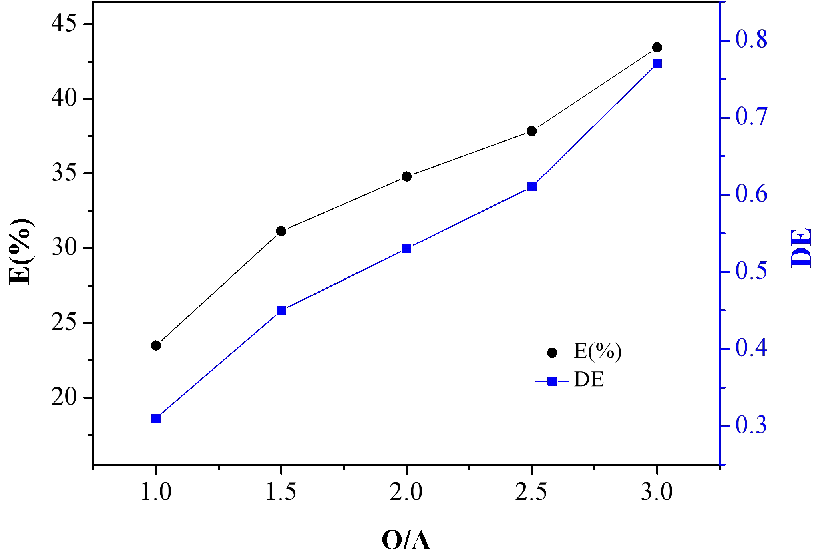
Figure 6. Effect of O/A on extraction of lactic acid.
3.3. Countercurrent extraction
From the results of single-stage extraction, it is concluded that the extraction effect of lactic acid is limited and it will consume a large amount of extractant. Consequently, multi-stage countercurrent extraction process is an effective means to increase the extraction rate of lactic acid in the extraction process.
The data of countercurrent extraction with 60 wt% N4423, 40 wt% 3# white oil and O/A=3 is shown in Table 2. The results showed that the good performance of N4423 at separating lactic and malic acids selectively was aimed at. This is confirmed experimentally that the value of β is greater than 1.3 in each round of countercurrent extraction. In addition, it reflectes a substantial improvement of the extractability of lactic and malic acids.
Table 2. Distribution coefficients (D) and separation factors (β) of lactic and malic acids
|
Roundnumber |
|
D |
|
β |
Roundnumber |
|
D |
|
β |
|
|
D1 |
|
D2 |
|
|
D1 |
|
D2 |
|
|
1 |
0.35 |
|
0.53 |
1.51 |
9 |
0.38 |
|
0.61 |
1.61 |
|
2 |
0.36 |
|
0.55 |
1.53 |
10 |
0.37 |
|
0.54 |
1.46 |
|
3 |
0.36 |
|
0.52 |
1.44 |
11 |
0.38 |
|
0.58 |
1.53 |
|
4 |
0.37 |
|
0.49 |
1.32 |
12 |
0.37 |
|
0.54 |
1.46 |
|
5 |
0.38 |
|
0.62 |
1.63 |
13 |
0.38 |
|
0.54 |
1.42 |
|
6 |
0.38 |
|
0.52 |
1.37 |
14 |
0.38 |
|
0.60 |
1.58 |
|
7 |
0.37 |
|
0.57 |
1.54 |
15 |
0.36 |
|
0.53 |
1.47 |
|
8 |
0.38 |
|
0.55 |
1.45 |
16 |
0.37 |
|
0.56 |
1.51 |
*D1---Back extraction distribution ratio of lactic acid.
D2---Back extraction distribution ratio of malic acid.
β-----Ratio between the distribution coefficients of lactic acid and malic acid.
3.4. Re-extraction of lactic and malic acids
In order to achieve high-purity lactic acid, a multi-stage countercurrent re-extraction process was adopted. The data of lactic acid stripping solution filtered by resin (Figure 7 (a)) and activated carbon (Figure 7 (b)) are presented. The solution for recovery of lactic and malic acids was double distilled water as it is economic, low energy consumption and it has hydrogen ion to directly take part in the decomplexation reaction (representated in eq3).
Figure 7 (a) illustrates that the results of lactic acid stripping solution that was filtered by resin. The following results can be drawn from the graph: the concentration of malic acid is 1.92 g/L, which peaks at 6.407 min and lactic acid has a purity of 95.19% which peaks at 7.272 min. Figure 7 (b) explained the details of lactic acid stripping solution which was filtered by activated carbon. The lactic acid has a purity of 98.78%, malic acid is 0.08 g/L. By comparing the above information, the activated carbon’s ability of decolorizing and adsorbing metal ions which contained in lactic acid stripping solution is better than resin. Hence, higher purity of lactic acid could be achieved. It is speculated that the substance with peaks at 5.5 min and 5.615 min would be gluconic acid, which its limited concentrations are 1.69 g/L (Figure 7 (a)) and 0.05 g /L (Figure 7 (b)).
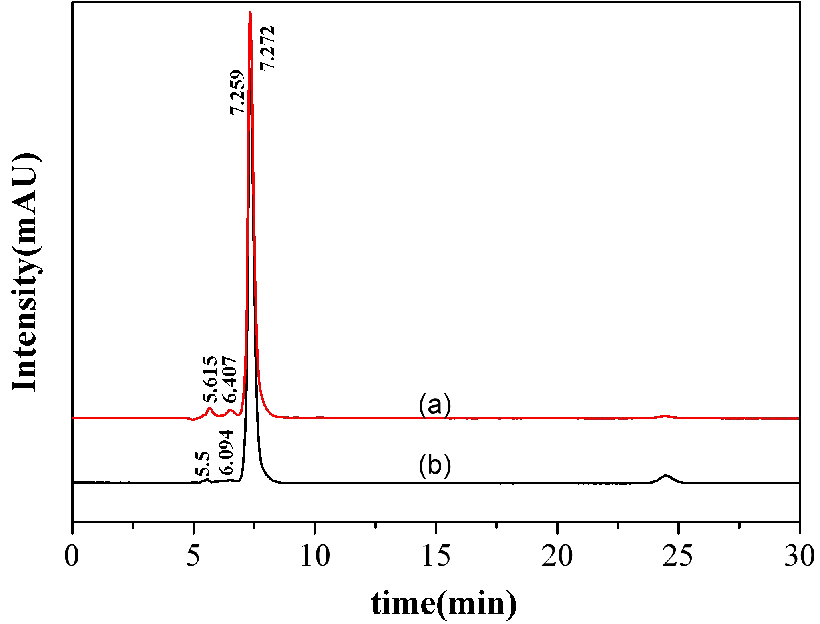
Figure 7. HPLC data of lactic acid stripping aqueous diluting 10 times filtered by resin (a) and activated carbon (b).
3.5. Separation of lactic and malic acids
In order to compare the degree of extraction and separation of lactic and malic acids with N4423, it is essential to have taken the following experiment.
3.5.1. Effect of temperature on extraction of lactic and malic acids
The complexation reactions of acid and amine in the organic phase involve proton transfer or hydrogen bond formation. Generally speaking, extraction temperature affects the intermolecular forces and the mass transfer rate. The conspicuous effects of temperature in the range of 25°C–75°C of lactic and malic acids were compared in Figure 8. Obviously, it could be concluded that the values of E(%) (Figure 8 (A)) and D(E) (Figure 8 (B)) of lactic and malic acids were negatively correlated with temperature. It can be seen that the curves slope of E(%) and D(E) of lactic acid are steeper than malic acid, thus, the effect of temperature on lactic acid is greater than that of malic acid. What’s more, the values of E(%) and DE of lactic acid are always higher than those of malic acid with temperature increasing. It can be inferred that the extraction of lactic acid is more sensitive to temperature.
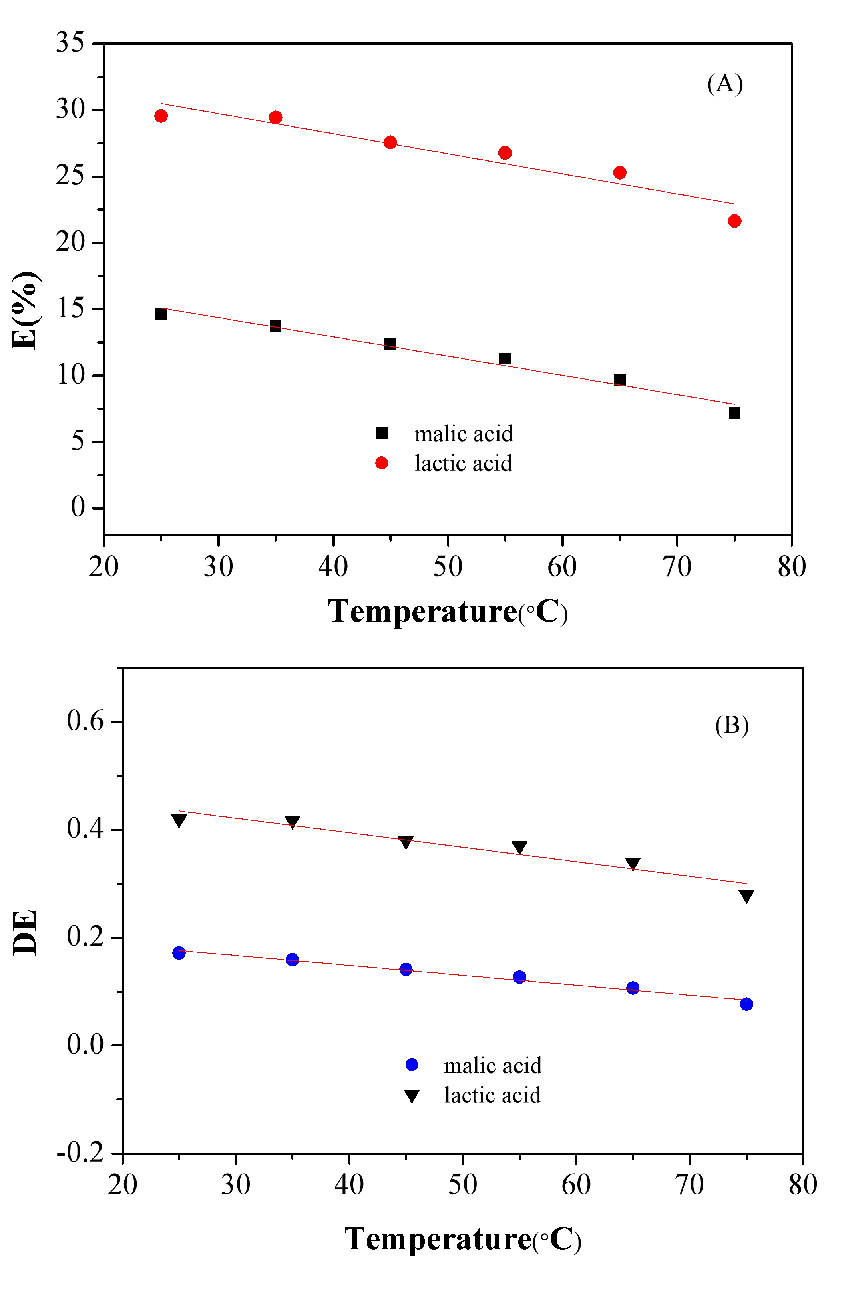
Figure 8. E (A) and DE (B) of lactic and malic acids in different extraction temperature.
3.5.3. Effect of lactic and malic acids concentrations
In order to explore the effect of lactic and malic acids concentration on the distribution ratio, several experiments were carried out. It could be shown by a linear plot of lgD vs lgCaq (Figure 9). It allowes the determination of the concentrations range of acids (0.01 mol/L-1 mol/L) in aqueous phase, at which one of the complexes formed in organic phase in equilibrium is present in low proportion. The data obtained in this work showes that the values of lgD of lactic acid are higher than those of malic acid, and nearly keeps constant with the rising of lactic acid concentration.The line slope of malic acid changes with acid concentration, showing positive slope for small concentration of malic acid, thus implying the favorable extraction of malic acid to organic phase in low acid concentration. According to Figure 9, the smaller the acid concentration, the steeper the tie-lines, and higher values of lgD would be achieved for both malic and lactic acids.
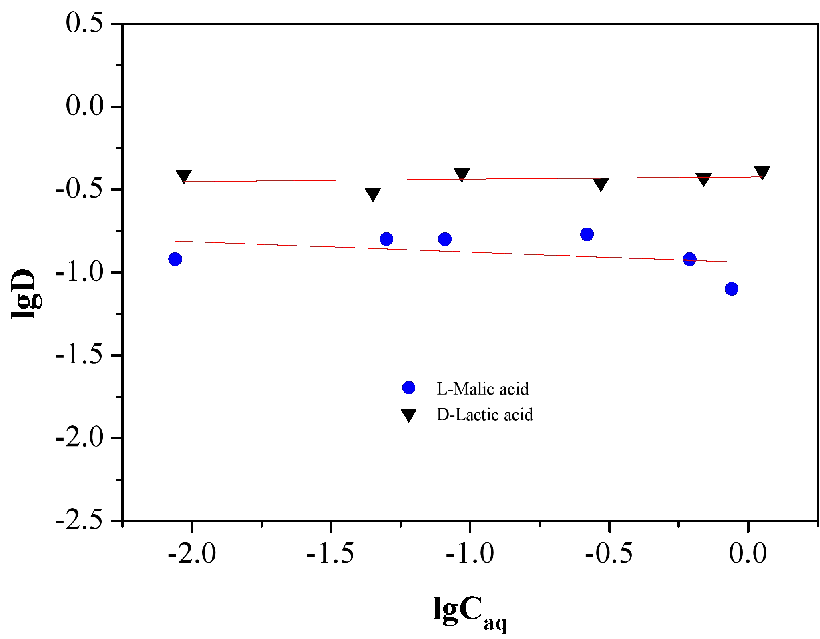
Figure 9. The lgD as a function of lgCaq.
3.6. Extraction equilibria
3.6.1. Study on extraction complex ratio
Experiments were carried out to describe the chemical equilibria for lactic and malic acids. Figure 10 shows the lnD as a function of ln[B] for a range of 30 wt%-80 wt% at 35℃. It was observed that a plot of lnD versus ln[B] yields a straight line with a slope of 1.61 and 1.86 of lactic and malic acids respectively, which implies that the equilibrium concentrations of lactic and malic acids in organic phase are high enough to form either the (1:1) or the (1:2) complex. For the experimental conditions used in this work, lactic acid concentration is high enough to provide the amount of lactic acid needed in organic phase so that the (1:2) complex can be formed, ie. one complex molecule might contain one N4423 molecule and two lactic acid molecules. Also, one complex molecule might contain one N4423 molecule and two malic acid molecules as the high enough concentration of malic acid in organic phase. However, it is understood that (1:2) complexation includes either hydrogen bonding (carbonyl complex) or ion pair formation (carboxylate complex), while, (1:1) complexation only involves hydrogen bonding formation. Critical comment on such work is rather difficult to give since Kex obtained here is only an overall constant and the temperature corrections of Kex are not considered here,36 thus, those values obtained at 35℃ were used.
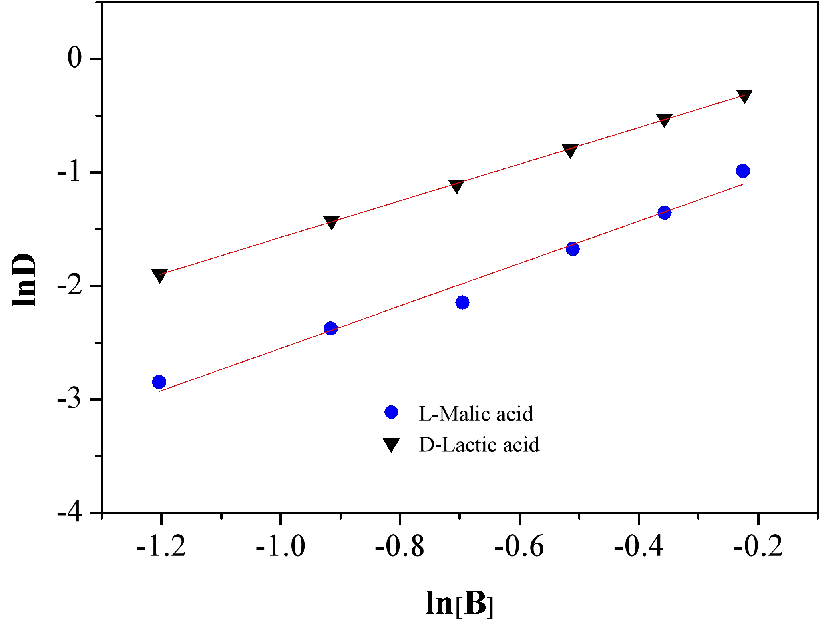
Figure 10. Relationship between ln[B] and lnD.
3.6.2. Thermodynamic equilibrium
In order to investigate the effect of temperature on the thermodynamic equilibrium of lactic and malic acids, the following experiments were performed. The equilibrium distribution coefficients were measured at 25℃-75℃, for 0.47 mol/L and 0.52 mol/L lactic and malic acids concentrations respectively, with 60 wt% N4423 in 40 wt% 3# white oil, and O/A=1. As is shown in Figure 11, It is observed that the best-fit formulation of the complexes is unchanged with temperature. It is surprising to find that the value of lnD is rather small at 25℃. This may imply that the contribution of (1:2) complex is greater as the temperature raised. The apparent enthalpies and entropies for the formation of complexes can be calculated as described in Table 3. It is found that the formation of (1:2) complex of lactic acid is less affected by temperature than malic acid. Also, (1:2) complex of malic acid is much more exothermic and involves a much greater loss of entropy than the formation of (1:2) complex of lactic acid. Therefore, the ability of N4423 to extract lactic acid is greater than malic acid, increasing the separation effect of lactic and malic acids.
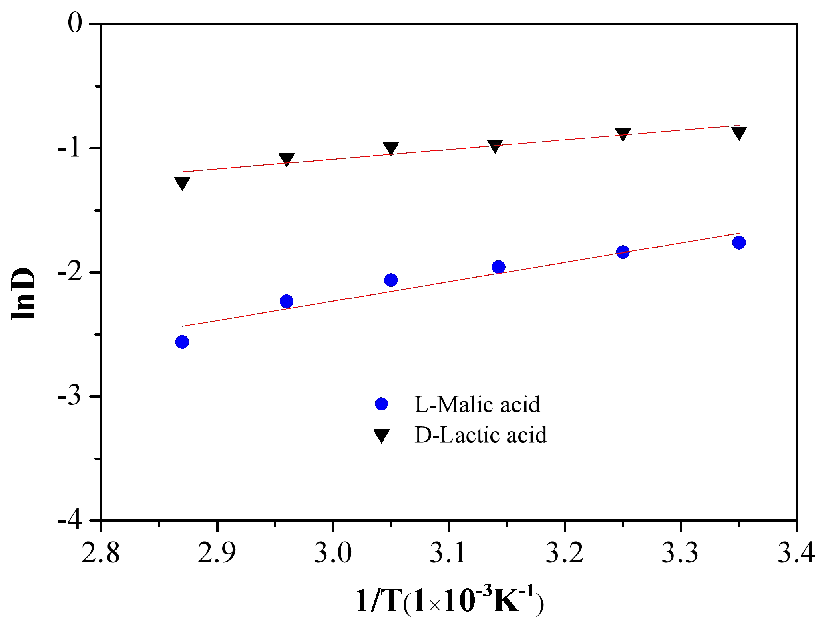
Figure 11. Relationship between 1/T and lnD.
Table 3. Apparent enthalpies and entropies for the formation of complexes.
|
Acid |
ΔH (KJ mol-1) |
ΔS (J mol-1 K-1) |
|
lactic acid |
-6.49 |
-21.06 |
|
malic acid |
-12.99 |
-47.83 |
3.6.3. Investigation of extraction mechanism
A commonly accepted extraction mechanism considers that the Lewis alkalinity of organic solvent containing O atom is responsible for the extraction efficiency, which can provide electron for forming hydrogen bonding with the extracted substrate.
In order to know clarify the extracting mechanism of lactic acid with N4423, FT-IR analysis of the extraction system was carried out. The results are shown in Figure 12. It was clear that with the addition of 3# white oil to N4423 system, the peak, corresponding to the stretching vibration of C=O (1629.04 cm-1) in N4423, had no significant change, implying the limited interaction between N4423 and 3# white oil. The stretching vibration of C=O in N4423 (1629.04 cm-1) showed an evident shift to lower wavenumber (1621.34 cm-1) with the extraction compound, proving the presence of interaction between N4423 and lactic acid molecules. When lactic acid was added to N4423, the stretching vibration of O-H in lactic acid (3410.39 cm-1) greatly shifted to higher wavenumber (3430.37 cm-1), therefore, the shift of the O-H stretching vibration suggests the existence of hydrogen bonding between N4423 and lactic acid during extraction species formation. This implied the formation of hydrogen bonding between H atom of lactic acid and O atom of N4423, which could be further proved by the fact that the stretching vibration of C=O in lactic acid (1720.89 cm-1) shifted to 1745.37 cm-1 in N4423 and lactic acid system, ascribing to the competed accepting of H in lactic acid between O atom of N4423 and C=O of lactic acid, so resulting in the breakage of intermolecular hydrogen bonding of lactic acid. In all, the formation of hydrogen bonding between O atom in N4423 and H of O-H in lactic acid is considered to be responsible for the high extraction efficiency of lactic acid which suggests that N4423 shows the best hydrogen-bond-accepting basicity (HBA) among the chosen organic solvents.
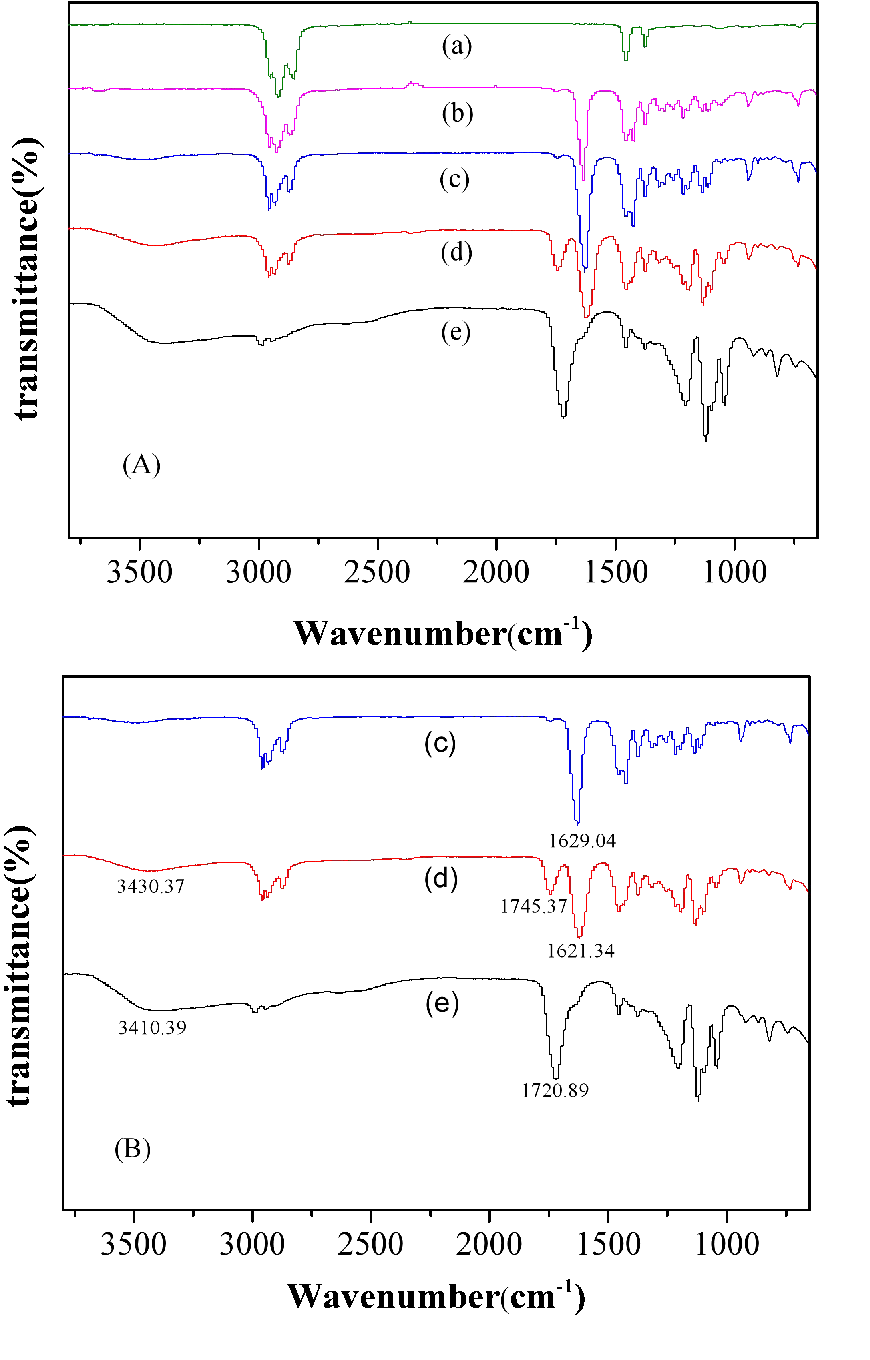
Figure 12. FT-IR spectra of 3# white oil (a), the mixture of N4423 and 3# white oil (b), N4423 (c), the mixture of N4423 and lactic acid (d), lactic acid (e).
The purpose of this study is to extract and separate the lactic and malic acids contained in fermentation broth in a high selective way which become increasingly significant. The extraction and separation processes of lactic and malic acids were successfully performed by using N4423/3# white oil extraction system and it is possible to recover the lactic and malic acids into double distilled water. To the best of our knowledge, this might be the first time to extract and separate lactic and malic acids from the fermentation solution directly derived from raw biomass via N4423. After recovering the lactic and malic acids, the organic solvent N4423 could be recycled with less than 10% loss and directly used for the next run.
In this paper, it shows that a composition of 60 wt% N4423 and 40 wt% 3# white oil is the optimum conditionsfor extraction of lactic acid. In addition, O/A=3, temperature at 35℃ and extraction time at 5 minutes are the optimal operating conditions. Based on the analysis by FT-IR, it is considered that the formation of hydrogen bonding between O atom of N4423 and H atom of O-H in lactic acid is responsible for the high extraction efficiency. This may provide an idea for exploring efficient, green and economical extractant. Due to the high efficiency for lactic acid extraction, N4423/3# white oil might be a potential extraction system for the separation and purification of lactic and malic acids from fermentation broth. Again, this results constitutes an orientation of the localization of the optimum conditions of extraction and separation of lactic and malic acids.
The authors gratefully acknowledge the Weihai Science and Technology Bureau (No. 2016DZJS04B06) for financial support of this project.
Dusselier M, Wouwe PV, Dewaele A, Makshina E and Sels BF, Lactic acid as a platform chemical in the biobased economy: the role of chemocatalysis. ENERG ENVIRON SCI 6:1415-1442 (2013).
View ArticleDatta R and Henry M, Lactic acid: recent advances in products, processes and technologies - a review. Journal of Chemical Technology & Biotechnology Biotechnology 81:1119-1129 (2010).
View ArticleMartinez FAC, Balciunas EM, Salgado JM, González JMD and Converti A, Lactic acid properties, applications and production: A review. TRENDS FOOD SCI TECH 30:70-83 (2013).
View ArticleSauer M, Porro D, Mattanovich D and Branduardi P, Microbial production of organic acids: expanding the markets. TRENDS BIOTECHNOL 26:100-108 (2008). PMid:18191255
View Article PubMed/NCBIP Ivi MKA, Simakova IL, Tapio S and Dmitry Yu M, Production of lactic acid/lactates from biomass and their catalytic transformations to commodities. CHEM REV 114:1909 (2014). PMid:24344682
View Article PubMed/NCBIChanukya BS, Kumar M and Rastogi NK, Optimization of lactic acid pertraction using liquid emulsion membranes by response surface methodology. Separation & Purification Technology 111:1-8 (2013).
View ArticleYordanov B and Boyadzhiev L, Pertraction of citric acid by means of emulsion liquid membranes. J MEMBRANE SCI 238:191-197 (2004).
View ArticleMarták J, Schlosser Š and Vlčková S, Pertraction of lactic acid through supported liquid membranes containing phosphonium ionic liquid. J MEMBRANE SCI 318:298-310 (2008).
View ArticleMadhumala M, Satyasri D, Sankarshana T and Sridhar S, Selective Extraction of Lactic Acid from Aqueous Media through a Hydrophobic H-Beta Zeolite/PVDF Mixed Matrix Membrane Contactor. IND ENG CHEM RES 53:17770-17781 (2014).
View ArticleVaidya AN, Pandey RA, Mudliar S, Kumar MS, Chakrabarti T and Devotta S, Production and Recovery of Lactic Acid for Polylactide-An Overview. Critical Reviews in Environmental Science & Technology 35:429-467 (2005).
View ArticleSosa AV, Ochoa J and Perotti NI, Modeling of direct recovery of lactic acid from whole broths by ion exchange adsorption. Bioseparation 9:283-289 (2000). PMid:11394567
View Article PubMed/NCBICockrem MCM, RECOVERY OF LACTATE ESTERS AND LACTIC ACID FROM FERMENTATION BROTH. (1993).
Sun X, Wang Q, Zhao W, Ma H and Sakata K, Extraction and purification of lactic acid from fermentation broth by esterification and hydrolysis method. Separation & Purification Technology 49:43-48 (2006).
View ArticleTao Z, The Study on Refining L-Lactic Acid by Molecular Distillation. Food & Fermentation Industries (2003).
Kyuchoukov G and Yankov D, Lactic Acid Extraction by Means of Long Chain Tertiary Amines: A Comparative Theoretical and Experimental Study. IND ENG CHEM RES 51:9117-9122 (2012).
View ArticleAimer M, Klemm E, Langanke B, Gehrke H and Stubenrauch C, Reactive Extraction of Lactic Acid by Using Tri-n-octylamine: Structure of the Ionic Phase. Chemistry 22:3268-3272 (2016). PMid:26821770
View Article PubMed/NCBIBi W, Zhou J and Row KH, Solid phase extraction of lactic acid from fermentation broth by anion-exchangeable silica confined ionic liquids. TALANTA 83:974-979 (2011). PMid:21147346
View Article PubMed/NCBIMatsumoto M, Takahashi T and Fukushima K, Synergistic extraction of lactic acid with alkylamine and tri- n -butylphosphate: effects of amines, diluents and temperature. Separation & Purification Technology 33:89-93 (2003). 00002-9
View ArticleMatsumoto M, Otono T and Kondo K, Synergistic extraction of organic acids with tri-n-octylamine and tri-n-butylphosphate. Separation & Purification Technology 24:337-342 (2001). 00137-X
View ArticleHossain MM and Maisuria JL, Effects of organic phase, fermentation media, and operating conditions on lactic Acid extraction. BIOTECHNOL PROGR 24:757-765 (2010). PMid:18376873
View Article PubMed/NCBIBreisig H, Schmidt M, Wolff H, Jupke A and Wessling M, Droplet-based liquid-liquid extraction inside a porous capillary. CHEM ENG J 307:143-149 (2017).
View ArticleWasewar KL, Shende D and Keshav A, Reactive Extraction of Itaconic Acid Using Quaternary Amine Aliquat 336 in Ethyl Acetate, Toluene, Hexane, and Kerosene. IND ENG CHEM RES 50:1003-1011 (2011).
View ArticleUslu H, Datta D, Santos D, Bamufleh HS and Bayat C, Separation of 2,4,6-trinitrophenol from aqueous solution by liquid-liquid extraction method: Equilibrium, kinetics, thermodynamics and molecular dynamic simulation. CHEM ENG J 299:342-352 (2016).
View ArticleKyuchoukov G, Labbaci A, Albet J and Molinier J, Simultaneous Influence of Active and Inert Diluents on the Extraction of Lactic Acid by Means of Tri-n-Octylamine (TOA) and Tri-Iso-Octylamine (TIOA). IND ENG CHEM RES 45:503-510 (2006).
View ArticleMatsumoto M, Mochiduki K, Fukunishi K and Kondo K, Extraction of organic acids using imidazolium-based ionic liquids and their toxicity to Lactobacillus rhamnosus. Separation & Purification Technology 40:97-101 (2004).
View ArticleWardell JM and King CJ, Solvent equilibriums for extraction of carboxylic acids from water. Journal of Chemical & Engineering Data 23:144-148 (1978).
View ArticleWennersten R, The Extraction of Citric-Acid From Fermentation Broth Using a Solution of a Tertiary Amine. Journal of Chemical Technology & Biotechnology 33:85-94 (2010).
View ArticleAnd RC and Eyal AM, Selectivity in Monocarboxylic Acids Extraction from Their Mixture Solutions Using an Amine-Based Extractant: Effect of pH. Ind.eng.chem.res 42:1301-1307 (2003).
View ArticleGeorge Kyuchoukov, Dragomir Yankov, Joël Albet A and Molinier J, Mechanism of Lactic Acid Extraction with Quaternary Ammonium Chloride (Aliquat 336). IND ENG CHEM RES 44:5733-5739 (2005).
View ArticleGeorge Kyuchoukov, Abdallah Labbaci, Joël Albet A and Molinier J, Simultaneous Influence of Active and "Inert" Diluents on the Extraction of Lactic Acid by Means of Tri-n-octylamine (TOA) and Tri-iso-octylamine (TIOA). IND ENG CHEM RES 45:503-510 (2006).
View ArticleWasewar KL, Yawalkar AA, Moulijn JA and Pangarkar VG, Fermentation of Glucose to Lactic Acid Coupled with Reactive Extraction: A Review. Ind.eng.chem.res 43 (2004).
View ArticleWasewar KL, Heesink AB, Versteeg GF and Pangarkar VG, Reactive extraction of lactic acid using alamine 336 in MIBK: equilibria and kinetics. J BIOTECHNOL 97:59-68 (2015). 00057-3
View ArticleKeshav A, Wasewar KL and Chand S, Extraction of propionic acid from model solutions: Effect of pH, salts, substrate, and temperature. AICHE J 55:1705-1711 (2010).
View ArticleKertes AS and King CJ, Extraction chemistry of fermentation product carboxylic acids. Biotechnology & Bioengineering 103:431-445 (2010). PMid:19388134
View Article PubMed/NCBIRatchford WP, Harris EH, Fisher CH and Willits CO, Extraction of Lactic Acid from Water Solution by Amine-Solvent Mixtures. Industrial & Engineering Chemistry 43:778-781.
View ArticleTamada JA and King CJ, Extraction of Carboxylic Acids with Amine Extractants.2. Chemical Interactions and Interpretation of Data. IND ENG CHEM RES 29:1327-1333 (1990).
View Article